Konversi Kg Ke M3
 How Can I Convert Emu Gm Into A M
How Can I Convert Emu Gm Into A M
Mengonversi liter/kilogram [L/kg] meter³/kilogram [m³/kg] • Mekanik • Volume Khusus • Kalkulator Ringkas

1 liter/kilogram [L/kg] = 0,001 meter³/kilogram [m³/kg]
The working fluid (steam) rotates the turbine of this gas-powered power station as it expands through it.
Heating, Ventilating, and Air Conditioning Technology
Pressure, Temperature, and Specific Volume
Specific Volume in Pressure Cookers
Gambaran umum
Refrigeration cycle. Step 1. The hot refrigerant, which was compressed by the compressor is cooled by outside air flow and condensed in this heat exchanger of a window air conditioner
Specific volume indicates volume per unit of mass — a property of substances and materials that is often used in thermodynamics. It is the inverse of density. To find specific volume we divide volume by mass. For gases it can also be found by using pressure, temperature, and the molecular mass. While volume per unit of mass is more common, sometimes specific volume may refer to volume per molecular weight. We can usually tell from the context which specific volume is used. The units for the specific volume by mass and specific volume by moles are different, so it is also possible to tell which specific volume is meant by looking at the units. Specific volume per mass is measured in m³/kg, L/kg, or ft³/lb and units derived from those, while molar volume is measured in m³/mol, and units derived from it. In some cases the latter is called molar volume or molar specific volume.
Applications
When we compare solids, liquids, and gases we will note that it is much easier to change density and specific volume for gases. Incidentally, while density is more commonly used when talking about liquids and solids, specific volume is more often used when working with gases. If we talk about systems that have both gas and a liquid within them, it is common to use specific volume when talking about both states of a substance.
Refrigeration cycle. Step 2. The cooled refrigerant in the liquid form is routed through the expansion valve and enters the evaporator heat exchanger shown in this picture. The warm room air is blown across the cold evaporator and is cooled there
Two-Phase Systems
Systems that have within them a substance in two different states of matter (liquid and gas, or liquid and solid) are known as two-phase systems. A mix of ice and water in a cup is one example. A system that has both liquid and vapor of this liquid in it, for example in a boiler of a gas-powered power station, or in a nuclear reactor, or in an air conditioner is another. We may want to observe how such a system behaves when temperature or pressure changes, in particular — whether the amount of substance in one phase increases or decreases as these parameters change. This is where we would use specific volume. To quantify properties of a two-phase system in general it is also convenient to use specific volume.
First let us consider some examples of two-phase systems and how they are used in our daily lives and in technology. After that we will discuss the relevance of specific volume.
Heating, Ventilating, and Air Conditioning Technology
Refrigeration cycle. Step 3. The gaseous refrigerant from the evaporator enters the compressor, is compressed there to a higher pressure and then enters the condenser heat exchanger and the refrigeration cycle is repeated
In many cases heating, ventilating, and air conditioning (HVAC) installations consist of a two-phase system. When the heating is on, it is sometimes the case that water is heated to produce steam, and the hot steam is then circulated through the pipes of the system to heat the room. Hot water is often used in many systems to carry the heat along the pipes. Some of these HVAC systems use boilers, which are heating devices. The water inside them is heated by burning some fuel, often the fossil fuel. Coal is commonly used, and so is natural gas.
When the air conditioning is in operation the air in the room is cooled by a substance called refrigerant that alternates between a gas and a liquid state. Initially this substance is cooled as a gas in the heat exchanger called condenser. When it cools, it condenses, meaning that it becomes a liquid. It is then sent by a compressor through the system to cool the room, and in this process it evaporates in another heat exchanger called evaporator and becomes a gas again, as it warms up. It is then returned to the first heat exchanger (condenser) to repeat the process.
Outside unit of a mini-split air conditioner system
Changing from liquid to gas requires much energy. During the cooling process the system absorbs energy from the room to heat the refrigerant, and in the process cools the room. The condenser in the air conditioner cools the gas by releasing the energy outside.
Kitchen and industrial refrigerators work using the same principle. Some of the HVAC systems work as a single unit, and some are a combination of a separate heater and an air conditioner.
Solar thermal collectors are used for heating
Solar Thermal Collectors
Thermal collectors within solar panels, in particular the flat plate collectors, work in a somewhat similar fashion as well. Solar energy enters the system through the front panel and heats air or liquid such as water or antifreeze. This thermal heat is used for heating or to provide hot water.
Heat pipes are highly efficient thermal conductors due to very high heat transfer during boiling and condensation of a liquid inside a heat pipe
Heat Pipe Systems
In a heat pipe system the process is similar to the air conditioner, except that it is not air that is cooled but surfaces made from various solid materials such as metals. The heat of these surfaces vaporizes the liquid in the heat pipe. The rest of the process is similar, with the gas condensing when cooled, and then returning back into the system. Some examples of cooling agents are helium, alcohol, and mercury. These systems are often used in electronic components especially in computers, to cool parts that are subject to rapid or extreme heating. They are also used in space in very extreme conditions.
How Two-Phase Systems Work
In a two-phase system there is usually a set of conditions in which both states can coexist within the system. If the properties of a substance is outside of the range of these conditions, then the substance can only be in one state, as we discuss in more detail below.
When we work with a two-phase system, it is the change in pressure, not in specific volume, that causes the temperature changes. However, this pressure change also changes the specific volume. In some situations we have a special case where the pressure and the temperature are constant, but the specific volume is changing. This happens if we have a system with constant pressure, at a temperature that allows two phases of the same substance to co-exist. In this case, once the system reaches this temperature, if it stays constant, the liquid within the system starts turning into gas and the specific volume increases as a result. Of course in this case the total volume of the system changes as well, so this is only possible if the system is flexible enough to accommodate that. In systems that have a set volume and mass and do not allow the specific volume to change, as we see in the example of a pressure cooker, the process is different. In our system the increase in specific volume will continue until the liquid evaporates and the system reaches equilibrium again.
The design of boilers and steam turbines used in power plants like the gas-powered plant shown in this picture requires a detailed understanding of two-phase flow heat transfer and pressure drop behaviour.
We just looked at what happens if we keep the pressure constant. Now let us consider the case when the temperature is constant and we increase the pressure. For each substance there is a specific range of pressure for which the substance can only exist in gas state. There is also a range of pressure for which the gas and the liquid state of the substance can exist together in the same system. As we change pressure, specific volume of the substance also changes.
Temperature also has a specific value above which the substance cannot coexist as a liquid and a gas in one system. This value for temperature is called critical temperature, and the value for pressure is called critical pressure. In thermodynamics, the critical temperature and the critical pressure combined are called a critical point.
In thermodynamics pressure, temperature, and specific volume are three values that form a special relationship. It is convenient to use them for describing a thermodynamic system because they are easy to measure. As we have discussed earlier, as long as we work with a single phase substance, a change in pressure or a change in temperature causes a decrease or an increase in specific volume. It depends on the substance, but for most gases increase in pressure means a decrease in specific volume, as long as we keep the temperature constant. On the other hand, increase in temperature results in increase in specific volume if the pressure is constant. This relationship means that if we control the specific volume, we can also control either pressure or temperature. This is the principle used in the pressure cooker.
In a pressure cooker, the boiling point of water increases up to 121 °C (250 °F) at the sea level at a pressure of 1 bar or ~15 psi above the existing atmospheric pressure
Specific Volume in Pressure Cookers
A pressure cooker is generally filled with food which is in liquid state. Often there is also some food in a solid state, but liquid is necessary for this type of cooking to work. When the lid is closed, the vapor cannot escape from the pressure cooker chamber except through the steam vent with the pressure regulator on it. Therefore during the cooking process the total volume is the same. The goal for pressure cooking is to cook the food inside at a higher temperature without allowing too much liquid to evaporate. This shortens the cooking time. We do need to generate some steam, however, because the additional goal that we try to achieve with a pressure cooker is to use hot steam for cooking. Steam has a much higher heat capacity than air, meaning that it is more efficient at storing energy. This is why it is used in pressure cooking. The energy efficiency of steam and the fact that we cook food at temperatures of about 120°С means that we can cook our food faster and use less energy to achieve the same result as we would with boiling or cooking in the oven.
We keep the mass and the volume of the contents of the pressure cooker more or less constant by preventing most of the steam from escaping. This ensures that the specific volume also stays somewhat constant. As we discussed earlier, if one among either the pressure, the temperature, or the specific volume is kept constant, then the magnitude of the other two variables depends on each other. Thus, if we increase temperature, as we do during pressure cooking, then the pressure inside increases also. Eventually we would reach the temperature-pressure balance, such that further increase in external temperature would result in evaporation. This is the maximum temperature that can be reached for the given specific volume and pressure. This is when we lower the heat to keep the temperature and the pressure constant for the remaining cooking time.
Using a pressure cooker is not only energy-efficient. It also reduces cooking times, as we mentioned before, and thus heats the kitchen a lot less, which is useful during the warm seasons. It can also be argued that pressure cooking is a healthy option because it does not require additional oil as does frying or stir-frying.
Artikel ini ditulis oleh Kateryna Yuri.
Anda kesulitan menerjemahkan satuan pengukuran ke bahasa lainnya? Bantuan tersedia! Posting pertanyaan Anda di TCTerms dan Anda akan mendapatkan jawaban dari penerjemah teknis berpengalaman dalam hitungan menit.
Page 2
1 liter/kilogram [L/kg] = 0,001 meter³/kilogram [m³/kg]
The working fluid (steam) rotates the turbine of this gas-powered power station as it expands through it.
Heating, Ventilating, and Air Conditioning Technology
Pressure, Temperature, and Specific Volume
Specific Volume in Pressure Cookers
Gambaran umum
Refrigeration cycle. Step 1. The hot refrigerant, which was compressed by the compressor is cooled by outside air flow and condensed in this heat exchanger of a window air conditioner
Specific volume indicates volume per unit of mass — a property of substances and materials that is often used in thermodynamics. It is the inverse of density. To find specific volume we divide volume by mass. For gases it can also be found by using pressure, temperature, and the molecular mass. While volume per unit of mass is more common, sometimes specific volume may refer to volume per molecular weight. We can usually tell from the context which specific volume is used. The units for the specific volume by mass and specific volume by moles are different, so it is also possible to tell which specific volume is meant by looking at the units. Specific volume per mass is measured in m³/kg, L/kg, or ft³/lb and units derived from those, while molar volume is measured in m³/mol, and units derived from it. In some cases the latter is called molar volume or molar specific volume.
Applications
When we compare solids, liquids, and gases we will note that it is much easier to change density and specific volume for gases. Incidentally, while density is more commonly used when talking about liquids and solids, specific volume is more often used when working with gases. If we talk about systems that have both gas and a liquid within them, it is common to use specific volume when talking about both states of a substance.
Refrigeration cycle. Step 2. The cooled refrigerant in the liquid form is routed through the expansion valve and enters the evaporator heat exchanger shown in this picture. The warm room air is blown across the cold evaporator and is cooled there
Two-Phase Systems
Systems that have within them a substance in two different states of matter (liquid and gas, or liquid and solid) are known as two-phase systems. A mix of ice and water in a cup is one example. A system that has both liquid and vapor of this liquid in it, for example in a boiler of a gas-powered power station, or in a nuclear reactor, or in an air conditioner is another. We may want to observe how such a system behaves when temperature or pressure changes, in particular — whether the amount of substance in one phase increases or decreases as these parameters change. This is where we would use specific volume. To quantify properties of a two-phase system in general it is also convenient to use specific volume.
First let us consider some examples of two-phase systems and how they are used in our daily lives and in technology. After that we will discuss the relevance of specific volume.
Heating, Ventilating, and Air Conditioning Technology
Refrigeration cycle. Step 3. The gaseous refrigerant from the evaporator enters the compressor, is compressed there to a higher pressure and then enters the condenser heat exchanger and the refrigeration cycle is repeated
In many cases heating, ventilating, and air conditioning (HVAC) installations consist of a two-phase system. When the heating is on, it is sometimes the case that water is heated to produce steam, and the hot steam is then circulated through the pipes of the system to heat the room. Hot water is often used in many systems to carry the heat along the pipes. Some of these HVAC systems use boilers, which are heating devices. The water inside them is heated by burning some fuel, often the fossil fuel. Coal is commonly used, and so is natural gas.
When the air conditioning is in operation the air in the room is cooled by a substance called refrigerant that alternates between a gas and a liquid state. Initially this substance is cooled as a gas in the heat exchanger called condenser. When it cools, it condenses, meaning that it becomes a liquid. It is then sent by a compressor through the system to cool the room, and in this process it evaporates in another heat exchanger called evaporator and becomes a gas again, as it warms up. It is then returned to the first heat exchanger (condenser) to repeat the process.
Outside unit of a mini-split air conditioner system
Changing from liquid to gas requires much energy. During the cooling process the system absorbs energy from the room to heat the refrigerant, and in the process cools the room. The condenser in the air conditioner cools the gas by releasing the energy outside.
Kitchen and industrial refrigerators work using the same principle. Some of the HVAC systems work as a single unit, and some are a combination of a separate heater and an air conditioner.
Solar thermal collectors are used for heating
Solar Thermal Collectors
Thermal collectors within solar panels, in particular the flat plate collectors, work in a somewhat similar fashion as well. Solar energy enters the system through the front panel and heats air or liquid such as water or antifreeze. This thermal heat is used for heating or to provide hot water.
Heat pipes are highly efficient thermal conductors due to very high heat transfer during boiling and condensation of a liquid inside a heat pipe
Heat Pipe Systems
In a heat pipe system the process is similar to the air conditioner, except that it is not air that is cooled but surfaces made from various solid materials such as metals. The heat of these surfaces vaporizes the liquid in the heat pipe. The rest of the process is similar, with the gas condensing when cooled, and then returning back into the system. Some examples of cooling agents are helium, alcohol, and mercury. These systems are often used in electronic components especially in computers, to cool parts that are subject to rapid or extreme heating. They are also used in space in very extreme conditions.
How Two-Phase Systems Work
In a two-phase system there is usually a set of conditions in which both states can coexist within the system. If the properties of a substance is outside of the range of these conditions, then the substance can only be in one state, as we discuss in more detail below.
When we work with a two-phase system, it is the change in pressure, not in specific volume, that causes the temperature changes. However, this pressure change also changes the specific volume. In some situations we have a special case where the pressure and the temperature are constant, but the specific volume is changing. This happens if we have a system with constant pressure, at a temperature that allows two phases of the same substance to co-exist. In this case, once the system reaches this temperature, if it stays constant, the liquid within the system starts turning into gas and the specific volume increases as a result. Of course in this case the total volume of the system changes as well, so this is only possible if the system is flexible enough to accommodate that. In systems that have a set volume and mass and do not allow the specific volume to change, as we see in the example of a pressure cooker, the process is different. In our system the increase in specific volume will continue until the liquid evaporates and the system reaches equilibrium again.
The design of boilers and steam turbines used in power plants like the gas-powered plant shown in this picture requires a detailed understanding of two-phase flow heat transfer and pressure drop behaviour.
We just looked at what happens if we keep the pressure constant. Now let us consider the case when the temperature is constant and we increase the pressure. For each substance there is a specific range of pressure for which the substance can only exist in gas state. There is also a range of pressure for which the gas and the liquid state of the substance can exist together in the same system. As we change pressure, specific volume of the substance also changes.
Temperature also has a specific value above which the substance cannot coexist as a liquid and a gas in one system. This value for temperature is called critical temperature, and the value for pressure is called critical pressure. In thermodynamics, the critical temperature and the critical pressure combined are called a critical point.
In thermodynamics pressure, temperature, and specific volume are three values that form a special relationship. It is convenient to use them for describing a thermodynamic system because they are easy to measure. As we have discussed earlier, as long as we work with a single phase substance, a change in pressure or a change in temperature causes a decrease or an increase in specific volume. It depends on the substance, but for most gases increase in pressure means a decrease in specific volume, as long as we keep the temperature constant. On the other hand, increase in temperature results in increase in specific volume if the pressure is constant. This relationship means that if we control the specific volume, we can also control either pressure or temperature. This is the principle used in the pressure cooker.
In a pressure cooker, the boiling point of water increases up to 121 °C (250 °F) at the sea level at a pressure of 1 bar or ~15 psi above the existing atmospheric pressure
Specific Volume in Pressure Cookers
A pressure cooker is generally filled with food which is in liquid state. Often there is also some food in a solid state, but liquid is necessary for this type of cooking to work. When the lid is closed, the vapor cannot escape from the pressure cooker chamber except through the steam vent with the pressure regulator on it. Therefore during the cooking process the total volume is the same. The goal for pressure cooking is to cook the food inside at a higher temperature without allowing too much liquid to evaporate. This shortens the cooking time. We do need to generate some steam, however, because the additional goal that we try to achieve with a pressure cooker is to use hot steam for cooking. Steam has a much higher heat capacity than air, meaning that it is more efficient at storing energy. This is why it is used in pressure cooking. The energy efficiency of steam and the fact that we cook food at temperatures of about 120°С means that we can cook our food faster and use less energy to achieve the same result as we would with boiling or cooking in the oven.
We keep the mass and the volume of the contents of the pressure cooker more or less constant by preventing most of the steam from escaping. This ensures that the specific volume also stays somewhat constant. As we discussed earlier, if one among either the pressure, the temperature, or the specific volume is kept constant, then the magnitude of the other two variables depends on each other. Thus, if we increase temperature, as we do during pressure cooking, then the pressure inside increases also. Eventually we would reach the temperature-pressure balance, such that further increase in external temperature would result in evaporation. This is the maximum temperature that can be reached for the given specific volume and pressure. This is when we lower the heat to keep the temperature and the pressure constant for the remaining cooking time.
Using a pressure cooker is not only energy-efficient. It also reduces cooking times, as we mentioned before, and thus heats the kitchen a lot less, which is useful during the warm seasons. It can also be argued that pressure cooking is a healthy option because it does not require additional oil as does frying or stir-frying.
Artikel ini ditulis oleh Kateryna Yuri.
Anda kesulitan menerjemahkan satuan pengukuran ke bahasa lainnya? Bantuan tersedia! Posting pertanyaan Anda di TCTerms dan Anda akan mendapatkan jawaban dari penerjemah teknis berpengalaman dalam hitungan menit.
Page 3
1 Liter/Kilogramm [l/kg] = 0,001 Kubikmeter/Kilogramm [m³/kg]
Das Arbeitsmedium (Dampf) bewegt die Turbine dieses gasbetriebenen Kraftwerks, während es sich ausdehnt.
Heizungs-, Belüftungs- und Klimaanlagentechnologie
Funktionsweise der Zweiphasensysteme
Druck, Temperatur und spezifisches Volumen
Spezifisches Volumen in Druckkochtöpfen
Überblick
Kühlzyklus. Schritt 1. Das erwärmte Kühlmittel, das durch den Kompressor komprimiert wurde, wird durch Luft von außen gekühlt und kondensiert in diesem Wärmetauscher einer Fensterklimaanlage
Spezifisches Volumen gibt das Volumen pro Einheit Masse an. Es ist eine Eigenschaft von Substanzen und Materialien, die häufig im Bereich der Thermodynamik verwendet werden. Es ist der Kehrwert der Dichte. Um spezifisches Volumen zu bestimmen, wird das Volumen durch die Masse dividiert. Bei Gasen kann es auch anhand von Druck, Temperatur und der molekularen Masse berechnet werden. Während Volumen pro Masseeinheit üblicher ist, bezieht sich das spezifische Volumen manchmal auf das Volumen pro Molekulargewicht. In der Regel sieht man am Kontext, welches spezifische Volumen genutzt wird. Die Einheiten für das spezifische Volumen nach Masse und das spezifische Volumen nach Mole sind unterschiedlich, also kann man die Art des spezifischen Volumens auch an der Einheit erkennen. Das spezifische Volumen pro Masse wird mit m³/kg, l/kg oder ft³/lb und Einheiten angegeben, die hiervon abgeleitet sind. Das Molvolumen wird mit m³/mol und hiervon abgeleiteten Einheiten angegeben. Es wird neben Molvolumen auch spezifisches Molvolumen genannt.
Anwendungen
Wenn man feste, flüssige und gasförmige Stoffe vergleicht, kann man feststellen, dass es für Gase einfacher ist, die Dichte und das spezifische Volumen zu ändern. Dichte wird häufiger in Bezug auf Flüssigkeiten und festen Stoffen verwendet; das spezifische Volumen findet mehr Anwendung bei der Arbeit mit Gasen. Bezogen auf Systeme, in denen sowohl Gase als auch Flüssigkeiten vorkommen, verwendet man üblicherweise spezifisches Volumen, wenn man über beide Zustände der Substanz spricht.
Kühlzyklus. Schritt 2. Das gekühlte Kühlmittel in flüssiger Form wird durch das Ausdehnungsventil geleitet und tritt in den Verdampfer ein. Die warme Raumluft strömt über den Verdampfer und wird gekühlt
Zweiphasensysteme
Systeme, in denen eine Substanz in zwei unterschiedlichen Zuständen (Flüssigkeit und Gas oder Flüssigkeit und Feststoff) vorkommt, werden Zweiphasensysteme genannt. Eine Mischung von Eis und Wasser in einem Becher ist ein Beispiel. Ein System, in dem sowohl Flüssigkeit als auch Dampf dieser Flüssigkeit enthalten sind, beispielsweise ein Kessel eines gasbetriebenen Kraftwerks, in einem Kernreaktor oder eine Klimaanlage, sind ebenfalls Zweiphasensysteme. Wenn wir beobachten möchten, wie ein solches System reagiert, wenn die Temperatur oder der Druck sich ändern, insbesondere ob die Menge der Substanz sich in einer Phase vergrößert oder verringert, sobald sich die Parameter ändern, dann würden wir das spezifische Volumen verwenden. Um die Eigenschaften in einem Zweiphasensystem allgemein zu quantifizieren, ist die Angabe als spezifisches Volumen ebenfalls komfortabel.
Betrachten wir zunächst Beispiele für Zweiphasensysteme und wie sie im Alltag und in der Technologie verwendet werden. Danach sprechen wir über die Bedeutung des spezifischen Volumens.
Heizungs-, Belüftungs- und Klimaanlagentechnologie
Kühlzyklus. Schritt 3. Das gasförmige Kühlmittel vom Verdampfer wandert in den Kompressor, wird dort auf einen höheren Druck komprimiert und zum Kondensator geleitet. Der Kühlzyklus beginnt von Neuem
In vielen Fällen bestehen Heizungs-, Belüftungs- und Klimaanlageninstallationen aus einem Zweiphasensystem. Bei einer Heizung wird in einigen Fällen Wasser erhitzt, um Dampf zu erzeugen. Der heiße Dampf wird dann durch Rohre des Systems geleitet, um den Raum zu heizen. Heißes Wasser wird in vielen Systemen verwendet, um Wärme in die Rohre zu transportieren. Einige dieser Heizungssysteme verwenden Kessel, in denen das Wasser erhitzt wird. Dafür wird häufig Brennstoff, meist fossile Brennstoffe, verfeuert. Kohle wird häufig genutzt, Erdgas ebenfalls.
Bei einer Klimaanlage wird der Raum von einer Substanz gekühlt, die Kühlmittel genannt wird und die zwischen einem gasförmigen und flüssigen Zustand wechselt. Erst wird die Substanz als Gas in einem Wärmetauscher, Kondensator genannt, gekühlt. Wenn das Gas abkühlt, kondensiert es und wird flüssig. Es wird dann von einem Kompressor durch das System geleitet, um den Raum zu kühlen. Bei diesem Vorgang verdampft es in einem weiteren Wärmetauscher, dem Verdampfer, und wird während seines Erwärmens wieder gasförmig. Dann kehrt es zum ersten Wärmetauscher (Kondensator) zurück und der Vorgang wiederholt sich.
Außeneinheit einer Mini-Split-Klimaanlage
Der Wechsel von Flüssigkeit nach Gas erfordert viel Energie. Während des Kühlungsprozesses absorbiert das System Energie aus dem Raum, um das Kühlmittel zu erwärmen. Dabei wird der Raum gekühlt. Der Kondensator der Klimaanlage kühlt das Gas, indem er die Energie nach außen abgibt.
Küchen- und Industriekühlschränke funktionieren nach dem gleichen Prinzip. Es gibt Systeme, die sowohl heizen als auch kühlen können.
Thermische Sonnenkollektoren werden für das Heizen verwendet
Thermische Sonnenkollektoren
Thermische Kollektoren in einem Solarmodul, insbesondere Flachkollektoren, arbeiten in gewisser Weise ähnlich. Sonnenenergie gelangt über die Module in das System und erhitzt Luft oder Flüssigkeit wie Wasser oder ein Frostschutzmittel. Diese thermische Hitze wird zum Heizen oder für heißes Wasser genutzt.
Wärmerohre sind sehr effiziente Wärmeleiter, da sie während des Erhitzens und der Kondensation von Flüssigkeit in einem Wärmerohr sehr große Hitze übertragen können
Wärmerohrleitungssystem
In einem Wärmerohrleitungssystem ist der Vorgang ähnlich zu dem einer Klimaanlage. Es wird jedoch nicht die Luft gekühlt, sondern Flächen aus verschiedenen festen Stoffen wie Metallen. Die Hitze der Fläche verdampft die Flüssigkeit im Wärmerohr. Der übrige Vorgang ist ähnlich: Das Gas kondensiert, wenn es gekühlt wird, und geht zurück in das System. Einige Beispiele für Kühlmittel sind Helium, Alkohol und Blei. Diese Systeme werden häufig in Elektrokomponenten, insbesondere Computern, verwendet, um Teile zu kühlen, die sonst schnell oder extrem aufheizen. Sie werden außerdem in der Raumfahrt unter sehr extremen Bedingungen genutzt.
Funktionsweise der Zweiphasensysteme
In einem Zweiphasensystem gibt es in der Regel eine Reihe von Bedingungen, unter denen beide Zustände im System nebeneinander existieren können. Wenn die Eigenschaften einer Substanz nicht im Rahmen dieser Bedingungen liegen, kann die Substanz nur in einem Zustand existieren.
Arbeitet man in einem Zweiphasensystem, ist es die Änderung am Druck, nicht am spezifischen Volumen, die eine Temperaturänderung bewirkt. Mit der Druckänderung verändert sich jedoch auch das spezifische Volumen. In einigen Situationen besteht ein Sonderfall, bei dem Druck und Temperatur konstant sind, aber das spezifische Volumen sich ändert. Dabei handelt es sich um ein System mit konstantem Druck bei einer Temperatur, die zwei Phasen der gleichen Substanz nebeneinander gestattet. Sobald das System diese Temperatur erreicht und sie konstant bleibt, wandelt sich die Flüssigkeit im System in Gas und das spezifische Volumen erhöht sich. In diesem Fall ändert sich das Gesamtvolumen des Systems ebenfalls, sodass der ganze Vorgang nur möglich ist, wenn das System hierfür flexibel genug ist. In einem System, bei dem Volumen und Masse feststehen und die nicht gestatten, dass sich das spezifische Volumen ändert, unterscheidet sich der Prozess, wie man am Beispiel eines Druckkochtopfs sehen kann. In diesem System wird die Erhöhung des spezifischen Volumens fortgesetzt, bis die Flüssigkeit verdampft und das System wieder ein Gleichgewicht erreicht.
Das Konzept von Kesseln und Dampfturbinen, die in Kraftwerken wie der gasbetriebenen Anlage im Bild verwendet werden, erfordern ein genaues Verständnis des Zweiphasenflusses der Wärmeübertragung und des Druckabfallverhaltens.
Dies ist also der Vorgang bei konstant bleibendem Druck. Was passiert, wenn die Temperatur konstant bleibt und der Druck erhöht wird? Für jede Substanz gibt es eine bestimmte Bandbreite des Drucks, bei dem die Substanz nur im Gaszustand existieren kann. Es gibt auch eine Bandbreite des Drucks, bei der der gasförmige und der flüssige Zustand in demselben System gleichzeitig existieren können. Wenn der Druck geändert wird, wird sich auch das spezifische Volumen der Substanz ändern.
Die Temperatur muss ebenfalls einem bestimmten Wert entsprechen, über dem die Substanz nicht gleichzeitig als Flüssigkeit und als Gas in einem System bestehen kann. Dieser Wert für Temperatur wird kritische Temperatur genannt; der Wert für Druck wird kritischer Druck genannt. In der Thermodynamik werden kritische Temperatur und kritischer Druck zusammen als kritischer Punkt bezeichnet.
In der Thermodynamik sind Druck, Temperatur und spezifisches Volumen drei Werte, die in einer besonderen Beziehung stehen. Durch ihre einfache Messung sind sie gut geeignet, ein thermodynamisches System zu beschreiben. Wie bereits erwähnt führt bei einer Einphasensubstanz die Änderung des Drucks oder der Temperatur zu einer Erhöhung oder Verringerung des spezifischen Volumens. Dies hängt von der Substanz ab, aber für die meisten Gase lässt sich sagen, dass eine Erhöhung des Drucks zu einer Verringerung des spezifischen Volumens führt, solang die Temperatur konstant bleibt. Andererseits führt eine Erhöhung der Temperatur zu einer Erhöhung des spezifischen Volumens, wenn der Druck konstant bleibt. Diese Beziehung bedeutet, die Möglichkeit sowohl Druck als auch Temperatur zu kontrollieren, wenn man das spezifische Volumen steuert. Das ist das Prinzip des Druckkochtopfs bzw. Dampfkochtopfs.
In einem Druckkochtopf erhöht sich der Siedepunkt von Wasser auf bis zu 121 °C (250 °F) auf Höhe des Meeresspiegels und bei einem Druck von 1 bar oder ~15 psi über dem bestehenden Atmosphärendruck
Spezifisches Volumen in Druckkochtöpfen
Ein Druckkochtopf wird in der Regel mit Lebensmitteln in flüssigem Zustand befüllt. Häufig sind auch feste Nahrungsmittel dabei, aber Flüssigkeit ist notwendig, damit das Kochen funktioniert. Wenn der Deckel geschlossen ist, kann kein Dampf aus dem Druckkochtopf außer durch das Dampfventil entweichen, an dem sich ein Druckregulator befindet. Daher ist während des Kochvorgangs das Gesamtvolumen gleich. Das Ziel beim Dampfgaren besteht darin, das Essen bei einer höheren Temperatur zu garen, wobei nicht zu viel Flüssigkeit verdampfen sollte. Das verkürzt die Kochzeit. Es muss jedoch eine bestimmte Menge Dampf erzeugt werden, denn das andere Ziel beim Kochen mit einem Druckkochtopf ist die Nutzung des heißen Dampfes zum Kochen. Dampf verfügt über eine viel höhere Hitzekapazität als Luft und kann Energie so effizienter speichern. Deshalb wird er beim Dampfgaren genutzt. Die Energieeffizienz von Dampf und die Tatsache, dass wir Essen bei Temperaturen von etwa 120 °С kochen, bedeutet, dass wir das Essen schneller kochen und weniger Energie benötigen, um das gleiche Ergebnis zu erzielen wie mit dem regulären Kochen.
Masse und Volumen der Inhalte des Druckkochtopfs bleiben in etwa konstant, indem verhindert wird, dass Dampf entweicht. Somit bleibt auch das spezifische Volumen nahzu gleich. Wenn wie erwähnt einer der Faktoren von Druck, Temperatur oder spezifischem Volumen konstant gehalten werden, hängt die Größe der anderen beiden Variablen voneinander ab. Wenn wir also die Temperatur erhöhen, wie das beim Dampfgaren der Fall ist, erhöht sich auch der Druck im Topf. Schließlich erreicht man einen Temperatur-Druck-Ausgleich, sodass eine weitere Erhöhung der externen Temperatur zum Verdampfen führen würde. Dies ist die erreichbare Höchstemperatur für ein bestimmtes spezifisches Volumen bei einem bestimmten Druck. Zu diesem Zeitpunkt verringern wir die Hitze, um die Temperatur und den Druck für die verbleibende Kochzeit konstant zu halten.
Der Einsatz eines Druckkochtopfs ist nicht nur energiesparend. Es verringert wie erwähnt auch die Kochzeit, somit wird die Küche selbst auch weniger erwärmt, was in heißen Monaten vorteilhaft sein kann. Für einige Menschen ergeben sich auch gesundheitliche Aspekte, da beim Dampfgaren kein Zusatz von Fett wie beim Frittieren oder Anbraten erforderlich ist.
Dieser Artikel wurde von Kateryna Yuri verfasst.
Haben Sie Schwierigkeiten, eine Messung in eine andere Sprache zu übersetzen? Hier erhalten Sie Hilfe! Stellen Sie Ihre Frage bei TCTerms und Sie erhalten von erfahrenen technischen Übersetzern binnen Minuten eine Antwort.
Page 4
1 liter/kilogram [L/kg] = 0.001 meter³/kilogram [m³/kg]
The working fluid (steam) rotates the turbine of this gas-powered power station as it expands through it.
Heating, Ventilating, and Air Conditioning Technology
Pressure, Temperature, and Specific Volume
Specific Volume in Pressure Cookers
Overview
Refrigeration cycle. Step 1. The hot refrigerant, which was compressed by the compressor is cooled by outside air flow and condensed in this heat exchanger of a window air conditioner
Specific volume indicates volume per unit of mass — a property of substances and materials that is often used in thermodynamics. It is the inverse of density. To find specific volume we divide volume by mass. For gases it can also be found by using pressure, temperature, and the molecular mass. While volume per unit of mass is more common, sometimes specific volume may refer to volume per molecular weight. We can usually tell from the context which specific volume is used. The units for the specific volume by mass and specific volume by moles are different, so it is also possible to tell which specific volume is meant by looking at the units. Specific volume per mass is measured in m³/kg, L/kg, or ft³/lb and units derived from those, while molar volume is measured in m³/mol, and units derived from it. In some cases the latter is called molar volume or molar specific volume.
Applications
When we compare solids, liquids, and gases we will note that it is much easier to change density and specific volume for gases. Incidentally, while density is more commonly used when talking about liquids and solids, specific volume is more often used when working with gases. If we talk about systems that have both gas and a liquid within them, it is common to use specific volume when talking about both states of a substance.
Refrigeration cycle. Step 2. The cooled refrigerant in the liquid form is routed through the expansion valve and enters the evaporator heat exchanger shown in this picture. The warm room air is blown across the cold evaporator and is cooled there
Two-Phase Systems
Systems that have within them a substance in two different states of matter (liquid and gas, or liquid and solid) are known as two-phase systems. A mix of ice and water in a cup is one example. A system that has both liquid and vapor of this liquid in it, for example in a boiler of a gas-powered power station, or in a nuclear reactor, or in an air conditioner is another. We may want to observe how such a system behaves when temperature or pressure changes, in particular — whether the amount of substance in one phase increases or decreases as these parameters change. This is where we would use specific volume. To quantify properties of a two-phase system in general it is also convenient to use specific volume.
First let us consider some examples of two-phase systems and how they are used in our daily lives and in technology. After that we will discuss the relevance of specific volume.
Heating, Ventilating, and Air Conditioning Technology
Refrigeration cycle. Step 3. The gaseous refrigerant from the evaporator enters the compressor, is compressed there to a higher pressure and then enters the condenser heat exchanger and the refrigeration cycle is repeated
In many cases heating, ventilating, and air conditioning (HVAC) installations consist of a two-phase system. When the heating is on, it is sometimes the case that water is heated to produce steam, and the hot steam is then circulated through the pipes of the system to heat the room. Hot water is often used in many systems to carry the heat along the pipes. Some of these HVAC systems use boilers, which are heating devices. The water inside them is heated by burning some fuel, often the fossil fuel. Coal is commonly used, and so is natural gas.
When the air conditioning is in operation the air in the room is cooled by a substance called refrigerant that alternates between a gas and a liquid state. Initially this substance is cooled as a gas in the heat exchanger called condenser. When it cools, it condenses, meaning that it becomes a liquid. It is then sent by a compressor through the system to cool the room, and in this process it evaporates in another heat exchanger called evaporator and becomes a gas again, as it warms up. It is then returned to the first heat exchanger (condenser) to repeat the process.
Outside unit of a mini-split air conditioner system
Changing from liquid to gas requires much energy. During the cooling process the system absorbs energy from the room to heat the refrigerant, and in the process cools the room. The condenser in the air conditioner cools the gas by releasing the energy outside.
Kitchen and industrial refrigerators work using the same principle. Some of the HVAC systems work as a single unit, and some are a combination of a separate heater and an air conditioner.
Solar thermal collectors are used for heating
Solar Thermal Collectors
Thermal collectors within solar panels, in particular the flat plate collectors, work in a somewhat similar fashion as well. Solar energy enters the system through the front panel and heats air or liquid such as water or antifreeze. This thermal heat is used for heating or to provide hot water.
Heat pipes are highly efficient thermal conductors due to very high heat transfer during boiling and condensation of a liquid inside a heat pipe
Heat Pipe Systems
In a heat pipe system the process is similar to the air conditioner, except that it is not air that is cooled but surfaces made from various solid materials such as metals. The heat of these surfaces vaporizes the liquid in the heat pipe. The rest of the process is similar, with the gas condensing when cooled, and then returning back into the system. Some examples of cooling agents are helium, alcohol, and mercury. These systems are often used in electronic components especially in computers, to cool parts that are subject to rapid or extreme heating. They are also used in space in very extreme conditions.
How Two-Phase Systems Work
In a two-phase system there is usually a set of conditions in which both states can coexist within the system. If the properties of a substance is outside of the range of these conditions, then the substance can only be in one state, as we discuss in more detail below.
When we work with a two-phase system, it is the change in pressure, not in specific volume, that causes the temperature changes. However, this pressure change also changes the specific volume. In some situations we have a special case where the pressure and the temperature are constant, but the specific volume is changing. This happens if we have a system with constant pressure, at a temperature that allows two phases of the same substance to co-exist. In this case, once the system reaches this temperature, if it stays constant, the liquid within the system starts turning into gas and the specific volume increases as a result. Of course in this case the total volume of the system changes as well, so this is only possible if the system is flexible enough to accommodate that. In systems that have a set volume and mass and do not allow the specific volume to change, as we see in the example of a pressure cooker, the process is different. In our system the increase in specific volume will continue until the liquid evaporates and the system reaches equilibrium again.
The design of boilers and steam turbines used in power plants like the gas-powered plant shown in this picture requires a detailed understanding of two-phase flow heat transfer and pressure drop behaviour.
We just looked at what happens if we keep the pressure constant. Now let us consider the case when the temperature is constant and we increase the pressure. For each substance there is a specific range of pressure for which the substance can only exist in gas state. There is also a range of pressure for which the gas and the liquid state of the substance can exist together in the same system. As we change pressure, specific volume of the substance also changes.
Temperature also has a specific value above which the substance cannot coexist as a liquid and a gas in one system. This value for temperature is called critical temperature, and the value for pressure is called critical pressure. In thermodynamics, the critical temperature and the critical pressure combined are called a critical point.
In thermodynamics pressure, temperature, and specific volume are three values that form a special relationship. It is convenient to use them for describing a thermodynamic system because they are easy to measure. As we have discussed earlier, as long as we work with a single phase substance, a change in pressure or a change in temperature causes a decrease or an increase in specific volume. It depends on the substance, but for most gases increase in pressure means a decrease in specific volume, as long as we keep the temperature constant. On the other hand, increase in temperature results in increase in specific volume if the pressure is constant. This relationship means that if we control the specific volume, we can also control either pressure or temperature. This is the principle used in the pressure cooker.
In a pressure cooker, the boiling point of water increases up to 121 °C (250 °F) at the sea level at a pressure of 1 bar or ~15 psi above the existing atmospheric pressure
Specific Volume in Pressure Cookers
A pressure cooker is generally filled with food which is in liquid state. Often there is also some food in a solid state, but liquid is necessary for this type of cooking to work. When the lid is closed, the vapor cannot escape from the pressure cooker chamber except through the steam vent with the pressure regulator on it. Therefore during the cooking process the total volume is the same. The goal for pressure cooking is to cook the food inside at a higher temperature without allowing too much liquid to evaporate. This shortens the cooking time. We do need to generate some steam, however, because the additional goal that we try to achieve with a pressure cooker is to use hot steam for cooking. Steam has a much higher heat capacity than air, meaning that it is more efficient at storing energy. This is why it is used in pressure cooking. The energy efficiency of steam and the fact that we cook food at temperatures of about 120°С means that we can cook our food faster and use less energy to achieve the same result as we would with boiling or cooking in the oven.
We keep the mass and the volume of the contents of the pressure cooker more or less constant by preventing most of the steam from escaping. This ensures that the specific volume also stays somewhat constant. As we discussed earlier, if one among either the pressure, the temperature, or the specific volume is kept constant, then the magnitude of the other two variables depends on each other. Thus, if we increase temperature, as we do during pressure cooking, then the pressure inside increases also. Eventually we would reach the temperature-pressure balance, such that further increase in external temperature would result in evaporation. This is the maximum temperature that can be reached for the given specific volume and pressure. This is when we lower the heat to keep the temperature and the pressure constant for the remaining cooking time.
Using a pressure cooker is not only energy-efficient. It also reduces cooking times, as we mentioned before, and thus heats the kitchen a lot less, which is useful during the warm seasons. It can also be argued that pressure cooking is a healthy option because it does not require additional oil as does frying or stir-frying.
This article was written by Kateryna Yuri
Do you have difficulty translating a measurement unit into another language? Help is available! Post your question in TCTerms and you will get an answer from experienced technical translators in minutes.
Page 5
1 liter/kilogram [L/kg] = 0,001 meter³/kilogram [m³/kg]
The working fluid (steam) rotates the turbine of this gas-powered power station as it expands through it.
Heating, Ventilating, and Air Conditioning Technology
Pressure, Temperature, and Specific Volume
Specific Volume in Pressure Cookers
Gambaran umum
Refrigeration cycle. Step 1. The hot refrigerant, which was compressed by the compressor is cooled by outside air flow and condensed in this heat exchanger of a window air conditioner
Specific volume indicates volume per unit of mass — a property of substances and materials that is often used in thermodynamics. It is the inverse of density. To find specific volume we divide volume by mass. For gases it can also be found by using pressure, temperature, and the molecular mass. While volume per unit of mass is more common, sometimes specific volume may refer to volume per molecular weight. We can usually tell from the context which specific volume is used. The units for the specific volume by mass and specific volume by moles are different, so it is also possible to tell which specific volume is meant by looking at the units. Specific volume per mass is measured in m³/kg, L/kg, or ft³/lb and units derived from those, while molar volume is measured in m³/mol, and units derived from it. In some cases the latter is called molar volume or molar specific volume.
Applications
When we compare solids, liquids, and gases we will note that it is much easier to change density and specific volume for gases. Incidentally, while density is more commonly used when talking about liquids and solids, specific volume is more often used when working with gases. If we talk about systems that have both gas and a liquid within them, it is common to use specific volume when talking about both states of a substance.
Refrigeration cycle. Step 2. The cooled refrigerant in the liquid form is routed through the expansion valve and enters the evaporator heat exchanger shown in this picture. The warm room air is blown across the cold evaporator and is cooled there
Two-Phase Systems
Systems that have within them a substance in two different states of matter (liquid and gas, or liquid and solid) are known as two-phase systems. A mix of ice and water in a cup is one example. A system that has both liquid and vapor of this liquid in it, for example in a boiler of a gas-powered power station, or in a nuclear reactor, or in an air conditioner is another. We may want to observe how such a system behaves when temperature or pressure changes, in particular — whether the amount of substance in one phase increases or decreases as these parameters change. This is where we would use specific volume. To quantify properties of a two-phase system in general it is also convenient to use specific volume.
First let us consider some examples of two-phase systems and how they are used in our daily lives and in technology. After that we will discuss the relevance of specific volume.
Heating, Ventilating, and Air Conditioning Technology
Refrigeration cycle. Step 3. The gaseous refrigerant from the evaporator enters the compressor, is compressed there to a higher pressure and then enters the condenser heat exchanger and the refrigeration cycle is repeated
In many cases heating, ventilating, and air conditioning (HVAC) installations consist of a two-phase system. When the heating is on, it is sometimes the case that water is heated to produce steam, and the hot steam is then circulated through the pipes of the system to heat the room. Hot water is often used in many systems to carry the heat along the pipes. Some of these HVAC systems use boilers, which are heating devices. The water inside them is heated by burning some fuel, often the fossil fuel. Coal is commonly used, and so is natural gas.
When the air conditioning is in operation the air in the room is cooled by a substance called refrigerant that alternates between a gas and a liquid state. Initially this substance is cooled as a gas in the heat exchanger called condenser. When it cools, it condenses, meaning that it becomes a liquid. It is then sent by a compressor through the system to cool the room, and in this process it evaporates in another heat exchanger called evaporator and becomes a gas again, as it warms up. It is then returned to the first heat exchanger (condenser) to repeat the process.
Outside unit of a mini-split air conditioner system
Changing from liquid to gas requires much energy. During the cooling process the system absorbs energy from the room to heat the refrigerant, and in the process cools the room. The condenser in the air conditioner cools the gas by releasing the energy outside.
Kitchen and industrial refrigerators work using the same principle. Some of the HVAC systems work as a single unit, and some are a combination of a separate heater and an air conditioner.
Solar thermal collectors are used for heating
Solar Thermal Collectors
Thermal collectors within solar panels, in particular the flat plate collectors, work in a somewhat similar fashion as well. Solar energy enters the system through the front panel and heats air or liquid such as water or antifreeze. This thermal heat is used for heating or to provide hot water.
Heat pipes are highly efficient thermal conductors due to very high heat transfer during boiling and condensation of a liquid inside a heat pipe
Heat Pipe Systems
In a heat pipe system the process is similar to the air conditioner, except that it is not air that is cooled but surfaces made from various solid materials such as metals. The heat of these surfaces vaporizes the liquid in the heat pipe. The rest of the process is similar, with the gas condensing when cooled, and then returning back into the system. Some examples of cooling agents are helium, alcohol, and mercury. These systems are often used in electronic components especially in computers, to cool parts that are subject to rapid or extreme heating. They are also used in space in very extreme conditions.
How Two-Phase Systems Work
In a two-phase system there is usually a set of conditions in which both states can coexist within the system. If the properties of a substance is outside of the range of these conditions, then the substance can only be in one state, as we discuss in more detail below.
When we work with a two-phase system, it is the change in pressure, not in specific volume, that causes the temperature changes. However, this pressure change also changes the specific volume. In some situations we have a special case where the pressure and the temperature are constant, but the specific volume is changing. This happens if we have a system with constant pressure, at a temperature that allows two phases of the same substance to co-exist. In this case, once the system reaches this temperature, if it stays constant, the liquid within the system starts turning into gas and the specific volume increases as a result. Of course in this case the total volume of the system changes as well, so this is only possible if the system is flexible enough to accommodate that. In systems that have a set volume and mass and do not allow the specific volume to change, as we see in the example of a pressure cooker, the process is different. In our system the increase in specific volume will continue until the liquid evaporates and the system reaches equilibrium again.
The design of boilers and steam turbines used in power plants like the gas-powered plant shown in this picture requires a detailed understanding of two-phase flow heat transfer and pressure drop behaviour.
We just looked at what happens if we keep the pressure constant. Now let us consider the case when the temperature is constant and we increase the pressure. For each substance there is a specific range of pressure for which the substance can only exist in gas state. There is also a range of pressure for which the gas and the liquid state of the substance can exist together in the same system. As we change pressure, specific volume of the substance also changes.
Temperature also has a specific value above which the substance cannot coexist as a liquid and a gas in one system. This value for temperature is called critical temperature, and the value for pressure is called critical pressure. In thermodynamics, the critical temperature and the critical pressure combined are called a critical point.
In thermodynamics pressure, temperature, and specific volume are three values that form a special relationship. It is convenient to use them for describing a thermodynamic system because they are easy to measure. As we have discussed earlier, as long as we work with a single phase substance, a change in pressure or a change in temperature causes a decrease or an increase in specific volume. It depends on the substance, but for most gases increase in pressure means a decrease in specific volume, as long as we keep the temperature constant. On the other hand, increase in temperature results in increase in specific volume if the pressure is constant. This relationship means that if we control the specific volume, we can also control either pressure or temperature. This is the principle used in the pressure cooker.
In a pressure cooker, the boiling point of water increases up to 121 °C (250 °F) at the sea level at a pressure of 1 bar or ~15 psi above the existing atmospheric pressure
Specific Volume in Pressure Cookers
A pressure cooker is generally filled with food which is in liquid state. Often there is also some food in a solid state, but liquid is necessary for this type of cooking to work. When the lid is closed, the vapor cannot escape from the pressure cooker chamber except through the steam vent with the pressure regulator on it. Therefore during the cooking process the total volume is the same. The goal for pressure cooking is to cook the food inside at a higher temperature without allowing too much liquid to evaporate. This shortens the cooking time. We do need to generate some steam, however, because the additional goal that we try to achieve with a pressure cooker is to use hot steam for cooking. Steam has a much higher heat capacity than air, meaning that it is more efficient at storing energy. This is why it is used in pressure cooking. The energy efficiency of steam and the fact that we cook food at temperatures of about 120°С means that we can cook our food faster and use less energy to achieve the same result as we would with boiling or cooking in the oven.
We keep the mass and the volume of the contents of the pressure cooker more or less constant by preventing most of the steam from escaping. This ensures that the specific volume also stays somewhat constant. As we discussed earlier, if one among either the pressure, the temperature, or the specific volume is kept constant, then the magnitude of the other two variables depends on each other. Thus, if we increase temperature, as we do during pressure cooking, then the pressure inside increases also. Eventually we would reach the temperature-pressure balance, such that further increase in external temperature would result in evaporation. This is the maximum temperature that can be reached for the given specific volume and pressure. This is when we lower the heat to keep the temperature and the pressure constant for the remaining cooking time.
Using a pressure cooker is not only energy-efficient. It also reduces cooking times, as we mentioned before, and thus heats the kitchen a lot less, which is useful during the warm seasons. It can also be argued that pressure cooking is a healthy option because it does not require additional oil as does frying or stir-frying.
Artikel ini ditulis oleh Kateryna Yuri.
Anda kesulitan menerjemahkan satuan pengukuran ke bahasa lainnya? Bantuan tersedia! Posting pertanyaan Anda di TCTerms dan Anda akan mendapatkan jawaban dari penerjemah teknis berpengalaman dalam hitungan menit.
Page 6
1 liter/kilogram [L/kg] = 0,001 meter³/kilogram [m³/kg]
The working fluid (steam) rotates the turbine of this gas-powered power station as it expands through it.
Heating, Ventilating, and Air Conditioning Technology
Pressure, Temperature, and Specific Volume
Specific Volume in Pressure Cookers
Overview
Refrigeration cycle. Step 1. The hot refrigerant, which was compressed by the compressor is cooled by outside air flow and condensed in this heat exchanger of a window air conditioner
Specific volume indicates volume per unit of mass — a property of substances and materials that is often used in thermodynamics. It is the inverse of density. To find specific volume we divide volume by mass. For gases it can also be found by using pressure, temperature, and the molecular mass. While volume per unit of mass is more common, sometimes specific volume may refer to volume per molecular weight. We can usually tell from the context which specific volume is used. The units for the specific volume by mass and specific volume by moles are different, so it is also possible to tell which specific volume is meant by looking at the units. Specific volume per mass is measured in m³/kg, L/kg, or ft³/lb and units derived from those, while molar volume is measured in m³/mol, and units derived from it. In some cases the latter is called molar volume or molar specific volume.
Applications
When we compare solids, liquids, and gases we will note that it is much easier to change density and specific volume for gases. Incidentally, while density is more commonly used when talking about liquids and solids, specific volume is more often used when working with gases. If we talk about systems that have both gas and a liquid within them, it is common to use specific volume when talking about both states of a substance.
Refrigeration cycle. Step 2. The cooled refrigerant in the liquid form is routed through the expansion valve and enters the evaporator heat exchanger shown in this picture. The warm room air is blown across the cold evaporator and is cooled there
Two-Phase Systems
Systems that have within them a substance in two different states of matter (liquid and gas, or liquid and solid) are known as two-phase systems. A mix of ice and water in a cup is one example. A system that has both liquid and vapor of this liquid in it, for example in a boiler of a gas-powered power station, or in a nuclear reactor, or in an air conditioner is another. We may want to observe how such a system behaves when temperature or pressure changes, in particular — whether the amount of substance in one phase increases or decreases as these parameters change. This is where we would use specific volume. To quantify properties of a two-phase system in general it is also convenient to use specific volume.
First let us consider some examples of two-phase systems and how they are used in our daily lives and in technology. After that we will discuss the relevance of specific volume.
Heating, Ventilating, and Air Conditioning Technology
Refrigeration cycle. Step 3. The gaseous refrigerant from the evaporator enters the compressor, is compressed there to a higher pressure and then enters the condenser heat exchanger and the refrigeration cycle is repeated
In many cases heating, ventilating, and air conditioning (HVAC) installations consist of a two-phase system. When the heating is on, it is sometimes the case that water is heated to produce steam, and the hot steam is then circulated through the pipes of the system to heat the room. Hot water is often used in many systems to carry the heat along the pipes. Some of these HVAC systems use boilers, which are heating devices. The water inside them is heated by burning some fuel, often the fossil fuel. Coal is commonly used, and so is natural gas.
When the air conditioning is in operation the air in the room is cooled by a substance called refrigerant that alternates between a gas and a liquid state. Initially this substance is cooled as a gas in the heat exchanger called condenser. When it cools, it condenses, meaning that it becomes a liquid. It is then sent by a compressor through the system to cool the room, and in this process it evaporates in another heat exchanger called evaporator and becomes a gas again, as it warms up. It is then returned to the first heat exchanger (condenser) to repeat the process.
Outside unit of a mini-split air conditioner system
Changing from liquid to gas requires much energy. During the cooling process the system absorbs energy from the room to heat the refrigerant, and in the process cools the room. The condenser in the air conditioner cools the gas by releasing the energy outside.
Kitchen and industrial refrigerators work using the same principle. Some of the HVAC systems work as a single unit, and some are a combination of a separate heater and an air conditioner.
Solar thermal collectors are used for heating
Solar Thermal Collectors
Thermal collectors within solar panels, in particular the flat plate collectors, work in a somewhat similar fashion as well. Solar energy enters the system through the front panel and heats air or liquid such as water or antifreeze. This thermal heat is used for heating or to provide hot water.
Heat pipes are highly efficient thermal conductors due to very high heat transfer during boiling and condensation of a liquid inside a heat pipe
Heat Pipe Systems
In a heat pipe system the process is similar to the air conditioner, except that it is not air that is cooled but surfaces made from various solid materials such as metals. The heat of these surfaces vaporizes the liquid in the heat pipe. The rest of the process is similar, with the gas condensing when cooled, and then returning back into the system. Some examples of cooling agents are helium, alcohol, and mercury. These systems are often used in electronic components especially in computers, to cool parts that are subject to rapid or extreme heating. They are also used in space in very extreme conditions.
How Two-Phase Systems Work
In a two-phase system there is usually a set of conditions in which both states can coexist within the system. If the properties of a substance is outside of the range of these conditions, then the substance can only be in one state, as we discuss in more detail below.
When we work with a two-phase system, it is the change in pressure, not in specific volume, that causes the temperature changes. However, this pressure change also changes the specific volume. In some situations we have a special case where the pressure and the temperature are constant, but the specific volume is changing. This happens if we have a system with constant pressure, at a temperature that allows two phases of the same substance to co-exist. In this case, once the system reaches this temperature, if it stays constant, the liquid within the system starts turning into gas and the specific volume increases as a result. Of course in this case the total volume of the system changes as well, so this is only possible if the system is flexible enough to accommodate that. In systems that have a set volume and mass and do not allow the specific volume to change, as we see in the example of a pressure cooker, the process is different. In our system the increase in specific volume will continue until the liquid evaporates and the system reaches equilibrium again.
The design of boilers and steam turbines used in power plants like the gas-powered plant shown in this picture requires a detailed understanding of two-phase flow heat transfer and pressure drop behaviour.
We just looked at what happens if we keep the pressure constant. Now let us consider the case when the temperature is constant and we increase the pressure. For each substance there is a specific range of pressure for which the substance can only exist in gas state. There is also a range of pressure for which the gas and the liquid state of the substance can exist together in the same system. As we change pressure, specific volume of the substance also changes.
Temperature also has a specific value above which the substance cannot coexist as a liquid and a gas in one system. This value for temperature is called critical temperature, and the value for pressure is called critical pressure. In thermodynamics, the critical temperature and the critical pressure combined are called a critical point.
In thermodynamics pressure, temperature, and specific volume are three values that form a special relationship. It is convenient to use them for describing a thermodynamic system because they are easy to measure. As we have discussed earlier, as long as we work with a single phase substance, a change in pressure or a change in temperature causes a decrease or an increase in specific volume. It depends on the substance, but for most gases increase in pressure means a decrease in specific volume, as long as we keep the temperature constant. On the other hand, increase in temperature results in increase in specific volume if the pressure is constant. This relationship means that if we control the specific volume, we can also control either pressure or temperature. This is the principle used in the pressure cooker.
In a pressure cooker, the boiling point of water increases up to 121 °C (250 °F) at the sea level at a pressure of 1 bar or ~15 psi above the existing atmospheric pressure
Specific Volume in Pressure Cookers
A pressure cooker is generally filled with food which is in liquid state. Often there is also some food in a solid state, but liquid is necessary for this type of cooking to work. When the lid is closed, the vapor cannot escape from the pressure cooker chamber except through the steam vent with the pressure regulator on it. Therefore during the cooking process the total volume is the same. The goal for pressure cooking is to cook the food inside at a higher temperature without allowing too much liquid to evaporate. This shortens the cooking time. We do need to generate some steam, however, because the additional goal that we try to achieve with a pressure cooker is to use hot steam for cooking. Steam has a much higher heat capacity than air, meaning that it is more efficient at storing energy. This is why it is used in pressure cooking. The energy efficiency of steam and the fact that we cook food at temperatures of about 120°С means that we can cook our food faster and use less energy to achieve the same result as we would with boiling or cooking in the oven.
We keep the mass and the volume of the contents of the pressure cooker more or less constant by preventing most of the steam from escaping. This ensures that the specific volume also stays somewhat constant. As we discussed earlier, if one among either the pressure, the temperature, or the specific volume is kept constant, then the magnitude of the other two variables depends on each other. Thus, if we increase temperature, as we do during pressure cooking, then the pressure inside increases also. Eventually we would reach the temperature-pressure balance, such that further increase in external temperature would result in evaporation. This is the maximum temperature that can be reached for the given specific volume and pressure. This is when we lower the heat to keep the temperature and the pressure constant for the remaining cooking time.
Using a pressure cooker is not only energy-efficient. It also reduces cooking times, as we mentioned before, and thus heats the kitchen a lot less, which is useful during the warm seasons. It can also be argued that pressure cooking is a healthy option because it does not require additional oil as does frying or stir-frying.
Questo articolo è stato scritto da Kateryna Yuri.
Hai trovato delle difficoltà nel tradurre un’unità di misura in un’altra lingua? Ti possiamo aiutare! Posta la tua questione nei TCTerms e riceverai le risposte di esperti traduttori tecnici in pochi minuti.
Page 7
1 литр на килограмм [л/кг] = 0,001 кубический метр на килограмм [м³/кг]
Вещество в парообразном состоянии расширяется и вращает турбины этой электростанции, которая работает на газе.
Использование удельного объема
Системы отопления, вентиляции и кондиционирования воздуха
Устройство и работа двухфазных систем
Температура, давление и удельный объем
Общие сведения
Цикл охлаждения, шаг 1. Горячий хладагент, сжатый компрессором, охлаждается окружающим воздухом и конденсируется в теплообменнике оконного кондиционера
Удельный объем — это объем на единицу массы. Это свойство веществ часто используется в термодинамике. Удельный объем — величина, обратная плотности. Его находят, разделив объем на массу. Удельный объем газов можно найти также по их плотности, температуре и молекулярной массе. Величину объема на единицу массы используют чаще, но иногда, говоря об удельном объеме, подразумевают отношение объема к молекулярной массе. Обычно из контекста понятно, о каком удельном объеме идет речь. Единицы удельного объема по массе отличаются от единиц удельного объема по молекулярной массе, поэтому можно понять, о каком удельном объеме идет речь, глядя на единицы, в которых эта величина измеряется. Удельный объем по массе измеряют в м³/кг, л/кг, или фут³/фунт, в то время как удельный объем по молекулярной массе измеряют в м³/моль и производных единицах. В некоторых случаях удельный объем по молекулярной массе называют молярным объемом или удельным молярным объемом.
Использование удельного объема
Если сравнить твердые вещества, жидкости и газы, то легко заметить, что изменить плотность или удельный объем газов проще всего. Кстати, когда говорят о твердых веществах и жидкостях, чаще всего используют плотность, а говоря о газах чаще используют удельный объем. Удельный объем также обычно используют при работе с системами, в которых вещество или вещества присутствуют в нескольких разных агрегатных состояниях.
Цикл охлаждения, шаг 2. Охлажденный хладагент в форме жидкости проходит через капиллярную трубку и попадает в испаритель (теплообменник, показанный на иллюстрации). Теплый воздух из комнаты проходит через холодный испаритель, где охлаждается
Двухфазные системы
Двухфазные системы — это системы, которые состоят из вещества, находящегося в двух разных агрегатных состояниях, например жидкость–газ, или жидкость–твердое тело. Смесь льда и воды в чашке — хороший пример системы жидкость–твердое тело. Системы жидкость–газ можно найти в котельной электростанции, которая работает на газе, в атомном реакторе или в кондиционере. В некоторых случаях интересно наблюдать за двухфазной системой, например чтобы узнать, как она изменяется при изменении температуры или давления. Нередко интерес представляют изменения в объеме вещества при изменении агрегатного состояния этого вещества. В этом случае используют удельный объем. В общем, удельный объем удобно использовать, чтобы описать свойства двухфазной системы.
Вначале рассмотрим примеры двухфазных систем и их применения в повседневной жизни и в технике. Затем обсудим применение удельного объема.
Системы отопления, вентиляции и кондиционирования воздуха
Цикл охлаждения, шаг 3. Хладагент в газообразном состоянии выходит из испарителя и попадает в компрессор, где его сжимают. При этом давление в хладагенте увеличивается. После он попадает в конденсатор (теплообменник), и цикл охлаждения повторяется
В большинстве установок отопления, вентиляции и кондиционирования (ОВК или по-английски HVAC) используются на двухфазные системы. При отоплении воду иногда нагревают до тех пор, пока она не превращается в пар, который подается по трубам системы отопления для нагрева помещения, конденсируется в радиаторах отопления в возвращается в котел в виде жидкости. Во многих системах отопления по трубам циркулирует горячая вода. В таких системы отопления для нагрева воды используют бойлеры. Воду в бойлере нагревают, сжигая топливо. Часто это ископаемое топливо, например уголь или природный газ.
С другой стороны, в процессе охлаждения используют вещество, называемое холодильным агентом или хладагентом. В процессе работы это вещество находится попеременно в двух фазах — жидкой и газообразной. Вначале газообразный хладагент охлаждают в теплообменнике, называемом конденсатором, до тех пор, пока он не переходит в жидкое состояние. Конденсатор находится вне охлаждаемого помещения. При этом хладагент конденсируется на стенках теплообменника, отдавая тепло в окружающую среду. После этого хладагент сжимают компрессором и пропускают по трубам через находящийся в охлаждаемом помещении другой теплообменник, называемый испарителем. В нем жидкий хладагент превращается в газ. На это преобразование требуется очень много тепла, которое и отбирается в охлаждаемом помещении. В газообразном состоянии хладагент возвращается в первый теплообменник, и весь процесс повторяется.
Уличный блок сплит-системы кондиционирования воздуха
Переход жидкости в газообразное состояние требует большое количество энергии. В процессе охлаждения система забирает тепло из комнаты для нагрева хладагента, и благодаря этому охлаждает помещение. Конденсатор в кондиционере охлаждает газ (хладагент), отдавая тепло в окружающую среду, то есть на улицу.
Домашние холодильники и промышленные холодильные камеры работают по такому же принципу. Некоторые устройства отопления, вентиляции и кондиционирования объединены в одну систему. В других случаях обогреватель и кондиционер представляют собой отдельные устройства.
Солнечные коллекторы используют для охлаждения
Солнечные коллекторы
Солнечные коллекторы работают по похожему принципу. Панели солнечных коллекторов собирают солнечную энергию, которая используется для нагрева воздуха или жидкости, например воды или антифриза. Полученную тепловую энергию используют для обогрева помещений или для нагрева воды.
Тепловые трубки — это высокоэффективные теплопередающее устройства. Их высокая теплопередача обеспечивается благодаря большому количеству энергии, которая расходуется на парообразование и выделяется при конденсации жидкости внутри них
Тепловые трубки
Процесс работы тепловых трубок похож на работу кондиционера, с разницей в том, что вместо охлаждения воздуха охлаждают твердые поверхности, например металлические. Тепло этих поверхностей нагревает жидкость в трубках до тех пор, пока эта жидкость не испаряется. В остальном процесс идентичен: газ охлаждается и конденсируется, и его снова возвращают в трубки для нагревания. Примеры охлаждающих веществ — это гелий, спирт, и ртуть. Нередко такие системы используют внутри электронных приборов, например компьютеров, для охлаждения электронных элементов, подверженных сильному нагреванию. Также эти системы используют в космосе в экстремальных температурных условиях.
Устройство и работа двухфазных систем
При определенных условиях вещество в двухфазных системах обычно может находиться в этой системе одновременно в двух разных фазах. Если же эти условия не соблюдены, то вещество в системе может быть только в одном агрегатном состоянии, как мы подробно опишем ниже.
В двухфазных системах изменения температуры вызваны изменением давления, а не удельного объема. Иногда, наоборот, давление и температура постоянные, а удельный объем изменяется. Это происходит, когда при постоянном давлении в системе поддерживается температура, которая позволяет веществу существовать одновременно в двух фазах. При таких условиях, как только система достигает нужной температуры, если эта температура не изменяется, то жидкость постепенно переходит в газообразное состояние, и удельный объем в результате увеличивается. Конечно, при этом изменяется и общий объем вещества в системе. Сама система также должна быть рассчитана на такое увеличение объема. С другой стороны, в системах с ограниченным объемом и массой, где невозможно изменять удельный объем, ситуация выглядит иначе. Ниже мы рассмотрим принцип работы такой системы на примере скороварки. Но вернемся к нашей системе, которая допускает изменения в удельном объеме. Удельный объем в ней будет увеличиваться до тех пор, пока вся жидкость не испарится и система вновь не достигнет равновесия.
Чтобы спроектировать котлы и турбины, используемые в электростанциях, например работающих на природном газе, как на фотографии, необходимо понимание теплового обмена и изменения давления в двухфазных системах
Только что мы познакомились с системами с неизменным давлением. Теперь рассмотрим систему с неизменной температурой и изменяющимся давлением. Для каждого вещества существует диапазон давлений, при которых оно может находиться только в газообразном состоянии. Также существует диапазон давлений, при котором вещество может быть одновременно и жидкостью и газом. Стоит заметить, что при изменении давления изменяется также и удельный объем.
Порог, после которого вещество не может быть одновременно в двух агрегатных состояниях, существует также и для жидкости. Порог температуры называют критической температурой, а порог давления — критическим давлением. Сочетание температуры и давления, при которых исчезают различия в свойствах жидкой и газообразной фаз вещества, в термодинамике называют критической точкой.
В термодинамике давление, температура и удельный объем — три величины, связанные между собой и зависящие друг от друга. Так как эти величины легко найти, их удобно использовать для описания термодинамических систем. Как мы описали выше, если вещество находится в одной фазе, то изменение давления или изменение температуры вызывают увеличение или уменьшение удельного объема. Как этот удельный объем изменяется, зависит от вещества, но для большинства газов увеличение давления при постоянной температуре вызывает уменьшение удельного объема. С другой стороны, увеличение температуры при постоянном давлении чаще всего увеличивает удельный объем. Такая зависимость также позволяет контролировать давление или температуру при помощи изменения удельного объема. Именно по такому принципу и работает скороварка.
Температура кипения воды в скороварке увеличивается до 121 °C (250 °F) на уровне моря при давлении, которое выше атмосферного давления на уровне моря на 1 бар или примерно 15 фунтов на квадратный дюйм
Удельный объем в скороварке
Чаще всего еда в скороварке — в форме жидкости. Конечно, часто в скороварке находятся и продукты питания в твердом состоянии, например мясо и овощи, но для успешной работы скороварки необходима жидкость. Когда крышка скороварки плотно закрыта, пар выходит из нее только через специальный патрубок, на который надет регулятор давления. Поэтому во время приготовления пищи в скороварке легко поддерживать постоянный удельный объем, что и делается. Главная цель приготовления еды в скороварке — приготовить еду с использованием более высокой температуры, и с наименьшим испарением жидкости. Такой способ ускоряет процесс приготовления пищи. Некоторое количество пара нам, все же, необходимо, так как именно горячий пар используется в скороварке для тепловой обработки продуктов. Теплоемкость пара намного выше теплоемкости воздуха, то есть он намного лучше воздуха хранит энергию. Высокая теплоемкость пара и тот факт, что скороварка позволяет нам поддерживать в ней температуру до 120° С означает, что еда в ней готовится намного быстрее и с меньшими затратами энергии, чем если бы ее готовили в кипящей воде или в духовом шкафу.
Чтобы поддерживать массу и объем неизменными, из скороварки почти не выпускают пар во время приготовления пищи. Это также помогает поддерживать более-менее постоянный удельный объем. Как уже обсуждалось ранее, если давление, температура или удельный объем постоянны, то величина двух других переменных зависит друг от друга. То есть, при увеличении температуры, как в начальной стадии приготовления пищи в скороварке, давление внутри скороварки также увеличится. Через некоторое время система достигнет равновесия между давлением и температурой. При дальнейшем увеличении наружной температуры жидкость в скороварке начнет испаряться. Эта температура — максимально возможная для данных давления и удельного объема. Как только наша система достигла этой температуры, мы убавляем огонь, чтобы поддерживать постоянную температуру и давление до конца процесса приготовления пищи.
Использование скороварки не только позволяет сберечь электроэнергию. Как мы упоминали выше, время приготовления пищи в скороварке намного меньше, чем если бы мы использовали другие методы приготовления, поэтому кухня нагревается намного меньше, что особенно важно в жаркую погоду. К тому же, еда, приготовленная в скороварке, намного полезнее для здоровья, чем, например, жареная еда, так как в скороварке не нужно масло, которое необходимо для жарения.
Автор статьи: Kateryna Yuri
Вы затрудняетесь в переводе единицы измерения с одного языка на другой? Коллеги готовы вам помочь. Опубликуйте вопрос в TCTerms и в течение нескольких минут вы получите ответ.
Page 8
1 литр на килограмм [л/кг] = 0,001 кубический метр на килограмм [м³/кг]
Вещество в парообразном состоянии расширяется и вращает турбины этой электростанции, которая работает на газе.
Использование удельного объема
Системы отопления, вентиляции и кондиционирования воздуха
Устройство и работа двухфазных систем
Температура, давление и удельный объем
Общие сведения
Цикл охлаждения, шаг 1. Горячий хладагент, сжатый компрессором, охлаждается окружающим воздухом и конденсируется в теплообменнике оконного кондиционера
Удельный объем — это объем на единицу массы. Это свойство веществ часто используется в термодинамике. Удельный объем — величина, обратная плотности. Его находят, разделив объем на массу. Удельный объем газов можно найти также по их плотности, температуре и молекулярной массе. Величину объема на единицу массы используют чаще, но иногда, говоря об удельном объеме, подразумевают отношение объема к молекулярной массе. Обычно из контекста понятно, о каком удельном объеме идет речь. Единицы удельного объема по массе отличаются от единиц удельного объема по молекулярной массе, поэтому можно понять, о каком удельном объеме идет речь, глядя на единицы, в которых эта величина измеряется. Удельный объем по массе измеряют в м³/кг, л/кг, или фут³/фунт, в то время как удельный объем по молекулярной массе измеряют в м³/моль и производных единицах. В некоторых случаях удельный объем по молекулярной массе называют молярным объемом или удельным молярным объемом.
Использование удельного объема
Если сравнить твердые вещества, жидкости и газы, то легко заметить, что изменить плотность или удельный объем газов проще всего. Кстати, когда говорят о твердых веществах и жидкостях, чаще всего используют плотность, а говоря о газах чаще используют удельный объем. Удельный объем также обычно используют при работе с системами, в которых вещество или вещества присутствуют в нескольких разных агрегатных состояниях.
Цикл охлаждения, шаг 2. Охлажденный хладагент в форме жидкости проходит через капиллярную трубку и попадает в испаритель (теплообменник, показанный на иллюстрации). Теплый воздух из комнаты проходит через холодный испаритель, где охлаждается
Двухфазные системы
Двухфазные системы — это системы, которые состоят из вещества, находящегося в двух разных агрегатных состояниях, например жидкость–газ, или жидкость–твердое тело. Смесь льда и воды в чашке — хороший пример системы жидкость–твердое тело. Системы жидкость–газ можно найти в котельной электростанции, которая работает на газе, в атомном реакторе или в кондиционере. В некоторых случаях интересно наблюдать за двухфазной системой, например чтобы узнать, как она изменяется при изменении температуры или давления. Нередко интерес представляют изменения в объеме вещества при изменении агрегатного состояния этого вещества. В этом случае используют удельный объем. В общем, удельный объем удобно использовать, чтобы описать свойства двухфазной системы.
Вначале рассмотрим примеры двухфазных систем и их применения в повседневной жизни и в технике. Затем обсудим применение удельного объема.
Системы отопления, вентиляции и кондиционирования воздуха
Цикл охлаждения, шаг 3. Хладагент в газообразном состоянии выходит из испарителя и попадает в компрессор, где его сжимают. При этом давление в хладагенте увеличивается. После он попадает в конденсатор (теплообменник), и цикл охлаждения повторяется
В большинстве установок отопления, вентиляции и кондиционирования (ОВК или по-английски HVAC) используются на двухфазные системы. При отоплении воду иногда нагревают до тех пор, пока она не превращается в пар, который подается по трубам системы отопления для нагрева помещения, конденсируется в радиаторах отопления в возвращается в котел в виде жидкости. Во многих системах отопления по трубам циркулирует горячая вода. В таких системы отопления для нагрева воды используют бойлеры. Воду в бойлере нагревают, сжигая топливо. Часто это ископаемое топливо, например уголь или природный газ.
С другой стороны, в процессе охлаждения используют вещество, называемое холодильным агентом или хладагентом. В процессе работы это вещество находится попеременно в двух фазах — жидкой и газообразной. Вначале газообразный хладагент охлаждают в теплообменнике, называемом конденсатором, до тех пор, пока он не переходит в жидкое состояние. Конденсатор находится вне охлаждаемого помещения. При этом хладагент конденсируется на стенках теплообменника, отдавая тепло в окружающую среду. После этого хладагент сжимают компрессором и пропускают по трубам через находящийся в охлаждаемом помещении другой теплообменник, называемый испарителем. В нем жидкий хладагент превращается в газ. На это преобразование требуется очень много тепла, которое и отбирается в охлаждаемом помещении. В газообразном состоянии хладагент возвращается в первый теплообменник, и весь процесс повторяется.
Уличный блок сплит-системы кондиционирования воздуха
Переход жидкости в газообразное состояние требует большое количество энергии. В процессе охлаждения система забирает тепло из комнаты для нагрева хладагента, и благодаря этому охлаждает помещение. Конденсатор в кондиционере охлаждает газ (хладагент), отдавая тепло в окружающую среду, то есть на улицу.
Домашние холодильники и промышленные холодильные камеры работают по такому же принципу. Некоторые устройства отопления, вентиляции и кондиционирования объединены в одну систему. В других случаях обогреватель и кондиционер представляют собой отдельные устройства.
Солнечные коллекторы используют для охлаждения
Солнечные коллекторы
Солнечные коллекторы работают по похожему принципу. Панели солнечных коллекторов собирают солнечную энергию, которая используется для нагрева воздуха или жидкости, например воды или антифриза. Полученную тепловую энергию используют для обогрева помещений или для нагрева воды.
Тепловые трубки — это высокоэффективные теплопередающее устройства. Их высокая теплопередача обеспечивается благодаря большому количеству энергии, которая расходуется на парообразование и выделяется при конденсации жидкости внутри них
Тепловые трубки
Процесс работы тепловых трубок похож на работу кондиционера, с разницей в том, что вместо охлаждения воздуха охлаждают твердые поверхности, например металлические. Тепло этих поверхностей нагревает жидкость в трубках до тех пор, пока эта жидкость не испаряется. В остальном процесс идентичен: газ охлаждается и конденсируется, и его снова возвращают в трубки для нагревания. Примеры охлаждающих веществ — это гелий, спирт, и ртуть. Нередко такие системы используют внутри электронных приборов, например компьютеров, для охлаждения электронных элементов, подверженных сильному нагреванию. Также эти системы используют в космосе в экстремальных температурных условиях.
Устройство и работа двухфазных систем
При определенных условиях вещество в двухфазных системах обычно может находиться в этой системе одновременно в двух разных фазах. Если же эти условия не соблюдены, то вещество в системе может быть только в одном агрегатном состоянии, как мы подробно опишем ниже.
В двухфазных системах изменения температуры вызваны изменением давления, а не удельного объема. Иногда, наоборот, давление и температура постоянные, а удельный объем изменяется. Это происходит, когда при постоянном давлении в системе поддерживается температура, которая позволяет веществу существовать одновременно в двух фазах. При таких условиях, как только система достигает нужной температуры, если эта температура не изменяется, то жидкость постепенно переходит в газообразное состояние, и удельный объем в результате увеличивается. Конечно, при этом изменяется и общий объем вещества в системе. Сама система также должна быть рассчитана на такое увеличение объема. С другой стороны, в системах с ограниченным объемом и массой, где невозможно изменять удельный объем, ситуация выглядит иначе. Ниже мы рассмотрим принцип работы такой системы на примере скороварки. Но вернемся к нашей системе, которая допускает изменения в удельном объеме. Удельный объем в ней будет увеличиваться до тех пор, пока вся жидкость не испарится и система вновь не достигнет равновесия.
Чтобы спроектировать котлы и турбины, используемые в электростанциях, например работающих на природном газе, как на фотографии, необходимо понимание теплового обмена и изменения давления в двухфазных системах
Только что мы познакомились с системами с неизменным давлением. Теперь рассмотрим систему с неизменной температурой и изменяющимся давлением. Для каждого вещества существует диапазон давлений, при которых оно может находиться только в газообразном состоянии. Также существует диапазон давлений, при котором вещество может быть одновременно и жидкостью и газом. Стоит заметить, что при изменении давления изменяется также и удельный объем.
Порог, после которого вещество не может быть одновременно в двух агрегатных состояниях, существует также и для жидкости. Порог температуры называют критической температурой, а порог давления — критическим давлением. Сочетание температуры и давления, при которых исчезают различия в свойствах жидкой и газообразной фаз вещества, в термодинамике называют критической точкой.
В термодинамике давление, температура и удельный объем — три величины, связанные между собой и зависящие друг от друга. Так как эти величины легко найти, их удобно использовать для описания термодинамических систем. Как мы описали выше, если вещество находится в одной фазе, то изменение давления или изменение температуры вызывают увеличение или уменьшение удельного объема. Как этот удельный объем изменяется, зависит от вещества, но для большинства газов увеличение давления при постоянной температуре вызывает уменьшение удельного объема. С другой стороны, увеличение температуры при постоянном давлении чаще всего увеличивает удельный объем. Такая зависимость также позволяет контролировать давление или температуру при помощи изменения удельного объема. Именно по такому принципу и работает скороварка.
Температура кипения воды в скороварке увеличивается до 121 °C (250 °F) на уровне моря при давлении, которое выше атмосферного давления на уровне моря на 1 бар или примерно 15 фунтов на квадратный дюйм
Удельный объем в скороварке
Чаще всего еда в скороварке — в форме жидкости. Конечно, часто в скороварке находятся и продукты питания в твердом состоянии, например мясо и овощи, но для успешной работы скороварки необходима жидкость. Когда крышка скороварки плотно закрыта, пар выходит из нее только через специальный патрубок, на который надет регулятор давления. Поэтому во время приготовления пищи в скороварке легко поддерживать постоянный удельный объем, что и делается. Главная цель приготовления еды в скороварке — приготовить еду с использованием более высокой температуры, и с наименьшим испарением жидкости. Такой способ ускоряет процесс приготовления пищи. Некоторое количество пара нам, все же, необходимо, так как именно горячий пар используется в скороварке для тепловой обработки продуктов. Теплоемкость пара намного выше теплоемкости воздуха, то есть он намного лучше воздуха хранит энергию. Высокая теплоемкость пара и тот факт, что скороварка позволяет нам поддерживать в ней температуру до 120° С означает, что еда в ней готовится намного быстрее и с меньшими затратами энергии, чем если бы ее готовили в кипящей воде или в духовом шкафу.
Чтобы поддерживать массу и объем неизменными, из скороварки почти не выпускают пар во время приготовления пищи. Это также помогает поддерживать более-менее постоянный удельный объем. Как уже обсуждалось ранее, если давление, температура или удельный объем постоянны, то величина двух других переменных зависит друг от друга. То есть, при увеличении температуры, как в начальной стадии приготовления пищи в скороварке, давление внутри скороварки также увеличится. Через некоторое время система достигнет равновесия между давлением и температурой. При дальнейшем увеличении наружной температуры жидкость в скороварке начнет испаряться. Эта температура — максимально возможная для данных давления и удельного объема. Как только наша система достигла этой температуры, мы убавляем огонь, чтобы поддерживать постоянную температуру и давление до конца процесса приготовления пищи.
Использование скороварки не только позволяет сберечь электроэнергию. Как мы упоминали выше, время приготовления пищи в скороварке намного меньше, чем если бы мы использовали другие методы приготовления, поэтому кухня нагревается намного меньше, что особенно важно в жаркую погоду. К тому же, еда, приготовленная в скороварке, намного полезнее для здоровья, чем, например, жареная еда, так как в скороварке не нужно масло, которое необходимо для жарения.
Автор статьи: Kateryna Yuri
Вы затрудняетесь в переводе единицы измерения с одного языка на другой? Коллеги готовы вам помочь. Опубликуйте вопрос в TCTerms и в течение нескольких минут вы получите ответ.
Page 9
1 liter/kilogram [L/kg] = 0.001 meter³/kilogram [m³/kg]
The working fluid (steam) rotates the turbine of this gas-powered power station as it expands through it.
Heating, Ventilating, and Air Conditioning Technology
Pressure, Temperature, and Specific Volume
Specific Volume in Pressure Cookers
Overview
Refrigeration cycle. Step 1. The hot refrigerant, which was compressed by the compressor is cooled by outside air flow and condensed in this heat exchanger of a window air conditioner
Specific volume indicates volume per unit of mass — a property of substances and materials that is often used in thermodynamics. It is the inverse of density. To find specific volume we divide volume by mass. For gases it can also be found by using pressure, temperature, and the molecular mass. While volume per unit of mass is more common, sometimes specific volume may refer to volume per molecular weight. We can usually tell from the context which specific volume is used. The units for the specific volume by mass and specific volume by moles are different, so it is also possible to tell which specific volume is meant by looking at the units. Specific volume per mass is measured in m³/kg, L/kg, or ft³/lb and units derived from those, while molar volume is measured in m³/mol, and units derived from it. In some cases the latter is called molar volume or molar specific volume.
Applications
When we compare solids, liquids, and gases we will note that it is much easier to change density and specific volume for gases. Incidentally, while density is more commonly used when talking about liquids and solids, specific volume is more often used when working with gases. If we talk about systems that have both gas and a liquid within them, it is common to use specific volume when talking about both states of a substance.
Refrigeration cycle. Step 2. The cooled refrigerant in the liquid form is routed through the expansion valve and enters the evaporator heat exchanger shown in this picture. The warm room air is blown across the cold evaporator and is cooled there
Two-Phase Systems
Systems that have within them a substance in two different states of matter (liquid and gas, or liquid and solid) are known as two-phase systems. A mix of ice and water in a cup is one example. A system that has both liquid and vapor of this liquid in it, for example in a boiler of a gas-powered power station, or in a nuclear reactor, or in an air conditioner is another. We may want to observe how such a system behaves when temperature or pressure changes, in particular — whether the amount of substance in one phase increases or decreases as these parameters change. This is where we would use specific volume. To quantify properties of a two-phase system in general it is also convenient to use specific volume.
First let us consider some examples of two-phase systems and how they are used in our daily lives and in technology. After that we will discuss the relevance of specific volume.
Heating, Ventilating, and Air Conditioning Technology
Refrigeration cycle. Step 3. The gaseous refrigerant from the evaporator enters the compressor, is compressed there to a higher pressure and then enters the condenser heat exchanger and the refrigeration cycle is repeated
In many cases heating, ventilating, and air conditioning (HVAC) installations consist of a two-phase system. When the heating is on, it is sometimes the case that water is heated to produce steam, and the hot steam is then circulated through the pipes of the system to heat the room. Hot water is often used in many systems to carry the heat along the pipes. Some of these HVAC systems use boilers, which are heating devices. The water inside them is heated by burning some fuel, often the fossil fuel. Coal is commonly used, and so is natural gas.
When the air conditioning is in operation the air in the room is cooled by a substance called refrigerant that alternates between a gas and a liquid state. Initially this substance is cooled as a gas in the heat exchanger called condenser. When it cools, it condenses, meaning that it becomes a liquid. It is then sent by a compressor through the system to cool the room, and in this process it evaporates in another heat exchanger called evaporator and becomes a gas again, as it warms up. It is then returned to the first heat exchanger (condenser) to repeat the process.
Outside unit of a mini-split air conditioner system
Changing from liquid to gas requires much energy. During the cooling process the system absorbs energy from the room to heat the refrigerant, and in the process cools the room. The condenser in the air conditioner cools the gas by releasing the energy outside.
Kitchen and industrial refrigerators work using the same principle. Some of the HVAC systems work as a single unit, and some are a combination of a separate heater and an air conditioner.
Solar thermal collectors are used for heating
Solar Thermal Collectors
Thermal collectors within solar panels, in particular the flat plate collectors, work in a somewhat similar fashion as well. Solar energy enters the system through the front panel and heats air or liquid such as water or antifreeze. This thermal heat is used for heating or to provide hot water.
Heat pipes are highly efficient thermal conductors due to very high heat transfer during boiling and condensation of a liquid inside a heat pipe
Heat Pipe Systems
In a heat pipe system the process is similar to the air conditioner, except that it is not air that is cooled but surfaces made from various solid materials such as metals. The heat of these surfaces vaporizes the liquid in the heat pipe. The rest of the process is similar, with the gas condensing when cooled, and then returning back into the system. Some examples of cooling agents are helium, alcohol, and mercury. These systems are often used in electronic components especially in computers, to cool parts that are subject to rapid or extreme heating. They are also used in space in very extreme conditions.
How Two-Phase Systems Work
In a two-phase system there is usually a set of conditions in which both states can coexist within the system. If the properties of a substance is outside of the range of these conditions, then the substance can only be in one state, as we discuss in more detail below.
When we work with a two-phase system, it is the change in pressure, not in specific volume, that causes the temperature changes. However, this pressure change also changes the specific volume. In some situations we have a special case where the pressure and the temperature are constant, but the specific volume is changing. This happens if we have a system with constant pressure, at a temperature that allows two phases of the same substance to co-exist. In this case, once the system reaches this temperature, if it stays constant, the liquid within the system starts turning into gas and the specific volume increases as a result. Of course in this case the total volume of the system changes as well, so this is only possible if the system is flexible enough to accommodate that. In systems that have a set volume and mass and do not allow the specific volume to change, as we see in the example of a pressure cooker, the process is different. In our system the increase in specific volume will continue until the liquid evaporates and the system reaches equilibrium again.
The design of boilers and steam turbines used in power plants like the gas-powered plant shown in this picture requires a detailed understanding of two-phase flow heat transfer and pressure drop behaviour.
We just looked at what happens if we keep the pressure constant. Now let us consider the case when the temperature is constant and we increase the pressure. For each substance there is a specific range of pressure for which the substance can only exist in gas state. There is also a range of pressure for which the gas and the liquid state of the substance can exist together in the same system. As we change pressure, specific volume of the substance also changes.
Temperature also has a specific value above which the substance cannot coexist as a liquid and a gas in one system. This value for temperature is called critical temperature, and the value for pressure is called critical pressure. In thermodynamics, the critical temperature and the critical pressure combined are called a critical point.
In thermodynamics pressure, temperature, and specific volume are three values that form a special relationship. It is convenient to use them for describing a thermodynamic system because they are easy to measure. As we have discussed earlier, as long as we work with a single phase substance, a change in pressure or a change in temperature causes a decrease or an increase in specific volume. It depends on the substance, but for most gases increase in pressure means a decrease in specific volume, as long as we keep the temperature constant. On the other hand, increase in temperature results in increase in specific volume if the pressure is constant. This relationship means that if we control the specific volume, we can also control either pressure or temperature. This is the principle used in the pressure cooker.
In a pressure cooker, the boiling point of water increases up to 121 °C (250 °F) at the sea level at a pressure of 1 bar or ~15 psi above the existing atmospheric pressure
Specific Volume in Pressure Cookers
A pressure cooker is generally filled with food which is in liquid state. Often there is also some food in a solid state, but liquid is necessary for this type of cooking to work. When the lid is closed, the vapor cannot escape from the pressure cooker chamber except through the steam vent with the pressure regulator on it. Therefore during the cooking process the total volume is the same. The goal for pressure cooking is to cook the food inside at a higher temperature without allowing too much liquid to evaporate. This shortens the cooking time. We do need to generate some steam, however, because the additional goal that we try to achieve with a pressure cooker is to use hot steam for cooking. Steam has a much higher heat capacity than air, meaning that it is more efficient at storing energy. This is why it is used in pressure cooking. The energy efficiency of steam and the fact that we cook food at temperatures of about 120°С means that we can cook our food faster and use less energy to achieve the same result as we would with boiling or cooking in the oven.
We keep the mass and the volume of the contents of the pressure cooker more or less constant by preventing most of the steam from escaping. This ensures that the specific volume also stays somewhat constant. As we discussed earlier, if one among either the pressure, the temperature, or the specific volume is kept constant, then the magnitude of the other two variables depends on each other. Thus, if we increase temperature, as we do during pressure cooking, then the pressure inside increases also. Eventually we would reach the temperature-pressure balance, such that further increase in external temperature would result in evaporation. This is the maximum temperature that can be reached for the given specific volume and pressure. This is when we lower the heat to keep the temperature and the pressure constant for the remaining cooking time.
Using a pressure cooker is not only energy-efficient. It also reduces cooking times, as we mentioned before, and thus heats the kitchen a lot less, which is useful during the warm seasons. It can also be argued that pressure cooking is a healthy option because it does not require additional oil as does frying or stir-frying.
This article was written by Kateryna Yuri
Do you have difficulty translating a measurement unit into another language? Help is available! Post your question in TCTerms and you will get an answer from experienced technical translators in minutes.
Page 10
1 Liter/Kilogramm [l/kg] = 0,001 Kubikmeter/Kilogramm [m³/kg]
Das Arbeitsmedium (Dampf) bewegt die Turbine dieses gasbetriebenen Kraftwerks, während es sich ausdehnt.
Heizungs-, Belüftungs- und Klimaanlagentechnologie
Funktionsweise der Zweiphasensysteme
Druck, Temperatur und spezifisches Volumen
Spezifisches Volumen in Druckkochtöpfen
Überblick
Kühlzyklus. Schritt 1. Das erwärmte Kühlmittel, das durch den Kompressor komprimiert wurde, wird durch Luft von außen gekühlt und kondensiert in diesem Wärmetauscher einer Fensterklimaanlage
Spezifisches Volumen gibt das Volumen pro Einheit Masse an. Es ist eine Eigenschaft von Substanzen und Materialien, die häufig im Bereich der Thermodynamik verwendet werden. Es ist der Kehrwert der Dichte. Um spezifisches Volumen zu bestimmen, wird das Volumen durch die Masse dividiert. Bei Gasen kann es auch anhand von Druck, Temperatur und der molekularen Masse berechnet werden. Während Volumen pro Masseeinheit üblicher ist, bezieht sich das spezifische Volumen manchmal auf das Volumen pro Molekulargewicht. In der Regel sieht man am Kontext, welches spezifische Volumen genutzt wird. Die Einheiten für das spezifische Volumen nach Masse und das spezifische Volumen nach Mole sind unterschiedlich, also kann man die Art des spezifischen Volumens auch an der Einheit erkennen. Das spezifische Volumen pro Masse wird mit m³/kg, l/kg oder ft³/lb und Einheiten angegeben, die hiervon abgeleitet sind. Das Molvolumen wird mit m³/mol und hiervon abgeleiteten Einheiten angegeben. Es wird neben Molvolumen auch spezifisches Molvolumen genannt.
Anwendungen
Wenn man feste, flüssige und gasförmige Stoffe vergleicht, kann man feststellen, dass es für Gase einfacher ist, die Dichte und das spezifische Volumen zu ändern. Dichte wird häufiger in Bezug auf Flüssigkeiten und festen Stoffen verwendet; das spezifische Volumen findet mehr Anwendung bei der Arbeit mit Gasen. Bezogen auf Systeme, in denen sowohl Gase als auch Flüssigkeiten vorkommen, verwendet man üblicherweise spezifisches Volumen, wenn man über beide Zustände der Substanz spricht.
Kühlzyklus. Schritt 2. Das gekühlte Kühlmittel in flüssiger Form wird durch das Ausdehnungsventil geleitet und tritt in den Verdampfer ein. Die warme Raumluft strömt über den Verdampfer und wird gekühlt
Zweiphasensysteme
Systeme, in denen eine Substanz in zwei unterschiedlichen Zuständen (Flüssigkeit und Gas oder Flüssigkeit und Feststoff) vorkommt, werden Zweiphasensysteme genannt. Eine Mischung von Eis und Wasser in einem Becher ist ein Beispiel. Ein System, in dem sowohl Flüssigkeit als auch Dampf dieser Flüssigkeit enthalten sind, beispielsweise ein Kessel eines gasbetriebenen Kraftwerks, in einem Kernreaktor oder eine Klimaanlage, sind ebenfalls Zweiphasensysteme. Wenn wir beobachten möchten, wie ein solches System reagiert, wenn die Temperatur oder der Druck sich ändern, insbesondere ob die Menge der Substanz sich in einer Phase vergrößert oder verringert, sobald sich die Parameter ändern, dann würden wir das spezifische Volumen verwenden. Um die Eigenschaften in einem Zweiphasensystem allgemein zu quantifizieren, ist die Angabe als spezifisches Volumen ebenfalls komfortabel.
Betrachten wir zunächst Beispiele für Zweiphasensysteme und wie sie im Alltag und in der Technologie verwendet werden. Danach sprechen wir über die Bedeutung des spezifischen Volumens.
Heizungs-, Belüftungs- und Klimaanlagentechnologie
Kühlzyklus. Schritt 3. Das gasförmige Kühlmittel vom Verdampfer wandert in den Kompressor, wird dort auf einen höheren Druck komprimiert und zum Kondensator geleitet. Der Kühlzyklus beginnt von Neuem
In vielen Fällen bestehen Heizungs-, Belüftungs- und Klimaanlageninstallationen aus einem Zweiphasensystem. Bei einer Heizung wird in einigen Fällen Wasser erhitzt, um Dampf zu erzeugen. Der heiße Dampf wird dann durch Rohre des Systems geleitet, um den Raum zu heizen. Heißes Wasser wird in vielen Systemen verwendet, um Wärme in die Rohre zu transportieren. Einige dieser Heizungssysteme verwenden Kessel, in denen das Wasser erhitzt wird. Dafür wird häufig Brennstoff, meist fossile Brennstoffe, verfeuert. Kohle wird häufig genutzt, Erdgas ebenfalls.
Bei einer Klimaanlage wird der Raum von einer Substanz gekühlt, die Kühlmittel genannt wird und die zwischen einem gasförmigen und flüssigen Zustand wechselt. Erst wird die Substanz als Gas in einem Wärmetauscher, Kondensator genannt, gekühlt. Wenn das Gas abkühlt, kondensiert es und wird flüssig. Es wird dann von einem Kompressor durch das System geleitet, um den Raum zu kühlen. Bei diesem Vorgang verdampft es in einem weiteren Wärmetauscher, dem Verdampfer, und wird während seines Erwärmens wieder gasförmig. Dann kehrt es zum ersten Wärmetauscher (Kondensator) zurück und der Vorgang wiederholt sich.
Außeneinheit einer Mini-Split-Klimaanlage
Der Wechsel von Flüssigkeit nach Gas erfordert viel Energie. Während des Kühlungsprozesses absorbiert das System Energie aus dem Raum, um das Kühlmittel zu erwärmen. Dabei wird der Raum gekühlt. Der Kondensator der Klimaanlage kühlt das Gas, indem er die Energie nach außen abgibt.
Küchen- und Industriekühlschränke funktionieren nach dem gleichen Prinzip. Es gibt Systeme, die sowohl heizen als auch kühlen können.
Thermische Sonnenkollektoren werden für das Heizen verwendet
Thermische Sonnenkollektoren
Thermische Kollektoren in einem Solarmodul, insbesondere Flachkollektoren, arbeiten in gewisser Weise ähnlich. Sonnenenergie gelangt über die Module in das System und erhitzt Luft oder Flüssigkeit wie Wasser oder ein Frostschutzmittel. Diese thermische Hitze wird zum Heizen oder für heißes Wasser genutzt.
Wärmerohre sind sehr effiziente Wärmeleiter, da sie während des Erhitzens und der Kondensation von Flüssigkeit in einem Wärmerohr sehr große Hitze übertragen können
Wärmerohrleitungssystem
In einem Wärmerohrleitungssystem ist der Vorgang ähnlich zu dem einer Klimaanlage. Es wird jedoch nicht die Luft gekühlt, sondern Flächen aus verschiedenen festen Stoffen wie Metallen. Die Hitze der Fläche verdampft die Flüssigkeit im Wärmerohr. Der übrige Vorgang ist ähnlich: Das Gas kondensiert, wenn es gekühlt wird, und geht zurück in das System. Einige Beispiele für Kühlmittel sind Helium, Alkohol und Blei. Diese Systeme werden häufig in Elektrokomponenten, insbesondere Computern, verwendet, um Teile zu kühlen, die sonst schnell oder extrem aufheizen. Sie werden außerdem in der Raumfahrt unter sehr extremen Bedingungen genutzt.
Funktionsweise der Zweiphasensysteme
In einem Zweiphasensystem gibt es in der Regel eine Reihe von Bedingungen, unter denen beide Zustände im System nebeneinander existieren können. Wenn die Eigenschaften einer Substanz nicht im Rahmen dieser Bedingungen liegen, kann die Substanz nur in einem Zustand existieren.
Arbeitet man in einem Zweiphasensystem, ist es die Änderung am Druck, nicht am spezifischen Volumen, die eine Temperaturänderung bewirkt. Mit der Druckänderung verändert sich jedoch auch das spezifische Volumen. In einigen Situationen besteht ein Sonderfall, bei dem Druck und Temperatur konstant sind, aber das spezifische Volumen sich ändert. Dabei handelt es sich um ein System mit konstantem Druck bei einer Temperatur, die zwei Phasen der gleichen Substanz nebeneinander gestattet. Sobald das System diese Temperatur erreicht und sie konstant bleibt, wandelt sich die Flüssigkeit im System in Gas und das spezifische Volumen erhöht sich. In diesem Fall ändert sich das Gesamtvolumen des Systems ebenfalls, sodass der ganze Vorgang nur möglich ist, wenn das System hierfür flexibel genug ist. In einem System, bei dem Volumen und Masse feststehen und die nicht gestatten, dass sich das spezifische Volumen ändert, unterscheidet sich der Prozess, wie man am Beispiel eines Druckkochtopfs sehen kann. In diesem System wird die Erhöhung des spezifischen Volumens fortgesetzt, bis die Flüssigkeit verdampft und das System wieder ein Gleichgewicht erreicht.
Das Konzept von Kesseln und Dampfturbinen, die in Kraftwerken wie der gasbetriebenen Anlage im Bild verwendet werden, erfordern ein genaues Verständnis des Zweiphasenflusses der Wärmeübertragung und des Druckabfallverhaltens.
Dies ist also der Vorgang bei konstant bleibendem Druck. Was passiert, wenn die Temperatur konstant bleibt und der Druck erhöht wird? Für jede Substanz gibt es eine bestimmte Bandbreite des Drucks, bei dem die Substanz nur im Gaszustand existieren kann. Es gibt auch eine Bandbreite des Drucks, bei der der gasförmige und der flüssige Zustand in demselben System gleichzeitig existieren können. Wenn der Druck geändert wird, wird sich auch das spezifische Volumen der Substanz ändern.
Die Temperatur muss ebenfalls einem bestimmten Wert entsprechen, über dem die Substanz nicht gleichzeitig als Flüssigkeit und als Gas in einem System bestehen kann. Dieser Wert für Temperatur wird kritische Temperatur genannt; der Wert für Druck wird kritischer Druck genannt. In der Thermodynamik werden kritische Temperatur und kritischer Druck zusammen als kritischer Punkt bezeichnet.
In der Thermodynamik sind Druck, Temperatur und spezifisches Volumen drei Werte, die in einer besonderen Beziehung stehen. Durch ihre einfache Messung sind sie gut geeignet, ein thermodynamisches System zu beschreiben. Wie bereits erwähnt führt bei einer Einphasensubstanz die Änderung des Drucks oder der Temperatur zu einer Erhöhung oder Verringerung des spezifischen Volumens. Dies hängt von der Substanz ab, aber für die meisten Gase lässt sich sagen, dass eine Erhöhung des Drucks zu einer Verringerung des spezifischen Volumens führt, solang die Temperatur konstant bleibt. Andererseits führt eine Erhöhung der Temperatur zu einer Erhöhung des spezifischen Volumens, wenn der Druck konstant bleibt. Diese Beziehung bedeutet, die Möglichkeit sowohl Druck als auch Temperatur zu kontrollieren, wenn man das spezifische Volumen steuert. Das ist das Prinzip des Druckkochtopfs bzw. Dampfkochtopfs.
In einem Druckkochtopf erhöht sich der Siedepunkt von Wasser auf bis zu 121 °C (250 °F) auf Höhe des Meeresspiegels und bei einem Druck von 1 bar oder ~15 psi über dem bestehenden Atmosphärendruck
Spezifisches Volumen in Druckkochtöpfen
Ein Druckkochtopf wird in der Regel mit Lebensmitteln in flüssigem Zustand befüllt. Häufig sind auch feste Nahrungsmittel dabei, aber Flüssigkeit ist notwendig, damit das Kochen funktioniert. Wenn der Deckel geschlossen ist, kann kein Dampf aus dem Druckkochtopf außer durch das Dampfventil entweichen, an dem sich ein Druckregulator befindet. Daher ist während des Kochvorgangs das Gesamtvolumen gleich. Das Ziel beim Dampfgaren besteht darin, das Essen bei einer höheren Temperatur zu garen, wobei nicht zu viel Flüssigkeit verdampfen sollte. Das verkürzt die Kochzeit. Es muss jedoch eine bestimmte Menge Dampf erzeugt werden, denn das andere Ziel beim Kochen mit einem Druckkochtopf ist die Nutzung des heißen Dampfes zum Kochen. Dampf verfügt über eine viel höhere Hitzekapazität als Luft und kann Energie so effizienter speichern. Deshalb wird er beim Dampfgaren genutzt. Die Energieeffizienz von Dampf und die Tatsache, dass wir Essen bei Temperaturen von etwa 120 °С kochen, bedeutet, dass wir das Essen schneller kochen und weniger Energie benötigen, um das gleiche Ergebnis zu erzielen wie mit dem regulären Kochen.
Masse und Volumen der Inhalte des Druckkochtopfs bleiben in etwa konstant, indem verhindert wird, dass Dampf entweicht. Somit bleibt auch das spezifische Volumen nahzu gleich. Wenn wie erwähnt einer der Faktoren von Druck, Temperatur oder spezifischem Volumen konstant gehalten werden, hängt die Größe der anderen beiden Variablen voneinander ab. Wenn wir also die Temperatur erhöhen, wie das beim Dampfgaren der Fall ist, erhöht sich auch der Druck im Topf. Schließlich erreicht man einen Temperatur-Druck-Ausgleich, sodass eine weitere Erhöhung der externen Temperatur zum Verdampfen führen würde. Dies ist die erreichbare Höchstemperatur für ein bestimmtes spezifisches Volumen bei einem bestimmten Druck. Zu diesem Zeitpunkt verringern wir die Hitze, um die Temperatur und den Druck für die verbleibende Kochzeit konstant zu halten.
Der Einsatz eines Druckkochtopfs ist nicht nur energiesparend. Es verringert wie erwähnt auch die Kochzeit, somit wird die Küche selbst auch weniger erwärmt, was in heißen Monaten vorteilhaft sein kann. Für einige Menschen ergeben sich auch gesundheitliche Aspekte, da beim Dampfgaren kein Zusatz von Fett wie beim Frittieren oder Anbraten erforderlich ist.
Dieser Artikel wurde von Kateryna Yuri verfasst.
Haben Sie Schwierigkeiten, eine Messung in eine andere Sprache zu übersetzen? Hier erhalten Sie Hilfe! Stellen Sie Ihre Frage bei TCTerms und Sie erhalten von erfahrenen technischen Übersetzern binnen Minuten eine Antwort.
Page 11
1 liter/kilogram [L/kg] = 0,001 meter³/kilogram [m³/kg]
The working fluid (steam) rotates the turbine of this gas-powered power station as it expands through it.
Heating, Ventilating, and Air Conditioning Technology
Pressure, Temperature, and Specific Volume
Specific Volume in Pressure Cookers
Gambaran umum
Refrigeration cycle. Step 1. The hot refrigerant, which was compressed by the compressor is cooled by outside air flow and condensed in this heat exchanger of a window air conditioner
Specific volume indicates volume per unit of mass — a property of substances and materials that is often used in thermodynamics. It is the inverse of density. To find specific volume we divide volume by mass. For gases it can also be found by using pressure, temperature, and the molecular mass. While volume per unit of mass is more common, sometimes specific volume may refer to volume per molecular weight. We can usually tell from the context which specific volume is used. The units for the specific volume by mass and specific volume by moles are different, so it is also possible to tell which specific volume is meant by looking at the units. Specific volume per mass is measured in m³/kg, L/kg, or ft³/lb and units derived from those, while molar volume is measured in m³/mol, and units derived from it. In some cases the latter is called molar volume or molar specific volume.
Applications
When we compare solids, liquids, and gases we will note that it is much easier to change density and specific volume for gases. Incidentally, while density is more commonly used when talking about liquids and solids, specific volume is more often used when working with gases. If we talk about systems that have both gas and a liquid within them, it is common to use specific volume when talking about both states of a substance.
Refrigeration cycle. Step 2. The cooled refrigerant in the liquid form is routed through the expansion valve and enters the evaporator heat exchanger shown in this picture. The warm room air is blown across the cold evaporator and is cooled there
Two-Phase Systems
Systems that have within them a substance in two different states of matter (liquid and gas, or liquid and solid) are known as two-phase systems. A mix of ice and water in a cup is one example. A system that has both liquid and vapor of this liquid in it, for example in a boiler of a gas-powered power station, or in a nuclear reactor, or in an air conditioner is another. We may want to observe how such a system behaves when temperature or pressure changes, in particular — whether the amount of substance in one phase increases or decreases as these parameters change. This is where we would use specific volume. To quantify properties of a two-phase system in general it is also convenient to use specific volume.
First let us consider some examples of two-phase systems and how they are used in our daily lives and in technology. After that we will discuss the relevance of specific volume.
Heating, Ventilating, and Air Conditioning Technology
Refrigeration cycle. Step 3. The gaseous refrigerant from the evaporator enters the compressor, is compressed there to a higher pressure and then enters the condenser heat exchanger and the refrigeration cycle is repeated
In many cases heating, ventilating, and air conditioning (HVAC) installations consist of a two-phase system. When the heating is on, it is sometimes the case that water is heated to produce steam, and the hot steam is then circulated through the pipes of the system to heat the room. Hot water is often used in many systems to carry the heat along the pipes. Some of these HVAC systems use boilers, which are heating devices. The water inside them is heated by burning some fuel, often the fossil fuel. Coal is commonly used, and so is natural gas.
When the air conditioning is in operation the air in the room is cooled by a substance called refrigerant that alternates between a gas and a liquid state. Initially this substance is cooled as a gas in the heat exchanger called condenser. When it cools, it condenses, meaning that it becomes a liquid. It is then sent by a compressor through the system to cool the room, and in this process it evaporates in another heat exchanger called evaporator and becomes a gas again, as it warms up. It is then returned to the first heat exchanger (condenser) to repeat the process.
Outside unit of a mini-split air conditioner system
Changing from liquid to gas requires much energy. During the cooling process the system absorbs energy from the room to heat the refrigerant, and in the process cools the room. The condenser in the air conditioner cools the gas by releasing the energy outside.
Kitchen and industrial refrigerators work using the same principle. Some of the HVAC systems work as a single unit, and some are a combination of a separate heater and an air conditioner.
Solar thermal collectors are used for heating
Solar Thermal Collectors
Thermal collectors within solar panels, in particular the flat plate collectors, work in a somewhat similar fashion as well. Solar energy enters the system through the front panel and heats air or liquid such as water or antifreeze. This thermal heat is used for heating or to provide hot water.
Heat pipes are highly efficient thermal conductors due to very high heat transfer during boiling and condensation of a liquid inside a heat pipe
Heat Pipe Systems
In a heat pipe system the process is similar to the air conditioner, except that it is not air that is cooled but surfaces made from various solid materials such as metals. The heat of these surfaces vaporizes the liquid in the heat pipe. The rest of the process is similar, with the gas condensing when cooled, and then returning back into the system. Some examples of cooling agents are helium, alcohol, and mercury. These systems are often used in electronic components especially in computers, to cool parts that are subject to rapid or extreme heating. They are also used in space in very extreme conditions.
How Two-Phase Systems Work
In a two-phase system there is usually a set of conditions in which both states can coexist within the system. If the properties of a substance is outside of the range of these conditions, then the substance can only be in one state, as we discuss in more detail below.
When we work with a two-phase system, it is the change in pressure, not in specific volume, that causes the temperature changes. However, this pressure change also changes the specific volume. In some situations we have a special case where the pressure and the temperature are constant, but the specific volume is changing. This happens if we have a system with constant pressure, at a temperature that allows two phases of the same substance to co-exist. In this case, once the system reaches this temperature, if it stays constant, the liquid within the system starts turning into gas and the specific volume increases as a result. Of course in this case the total volume of the system changes as well, so this is only possible if the system is flexible enough to accommodate that. In systems that have a set volume and mass and do not allow the specific volume to change, as we see in the example of a pressure cooker, the process is different. In our system the increase in specific volume will continue until the liquid evaporates and the system reaches equilibrium again.
The design of boilers and steam turbines used in power plants like the gas-powered plant shown in this picture requires a detailed understanding of two-phase flow heat transfer and pressure drop behaviour.
We just looked at what happens if we keep the pressure constant. Now let us consider the case when the temperature is constant and we increase the pressure. For each substance there is a specific range of pressure for which the substance can only exist in gas state. There is also a range of pressure for which the gas and the liquid state of the substance can exist together in the same system. As we change pressure, specific volume of the substance also changes.
Temperature also has a specific value above which the substance cannot coexist as a liquid and a gas in one system. This value for temperature is called critical temperature, and the value for pressure is called critical pressure. In thermodynamics, the critical temperature and the critical pressure combined are called a critical point.
In thermodynamics pressure, temperature, and specific volume are three values that form a special relationship. It is convenient to use them for describing a thermodynamic system because they are easy to measure. As we have discussed earlier, as long as we work with a single phase substance, a change in pressure or a change in temperature causes a decrease or an increase in specific volume. It depends on the substance, but for most gases increase in pressure means a decrease in specific volume, as long as we keep the temperature constant. On the other hand, increase in temperature results in increase in specific volume if the pressure is constant. This relationship means that if we control the specific volume, we can also control either pressure or temperature. This is the principle used in the pressure cooker.
In a pressure cooker, the boiling point of water increases up to 121 °C (250 °F) at the sea level at a pressure of 1 bar or ~15 psi above the existing atmospheric pressure
Specific Volume in Pressure Cookers
A pressure cooker is generally filled with food which is in liquid state. Often there is also some food in a solid state, but liquid is necessary for this type of cooking to work. When the lid is closed, the vapor cannot escape from the pressure cooker chamber except through the steam vent with the pressure regulator on it. Therefore during the cooking process the total volume is the same. The goal for pressure cooking is to cook the food inside at a higher temperature without allowing too much liquid to evaporate. This shortens the cooking time. We do need to generate some steam, however, because the additional goal that we try to achieve with a pressure cooker is to use hot steam for cooking. Steam has a much higher heat capacity than air, meaning that it is more efficient at storing energy. This is why it is used in pressure cooking. The energy efficiency of steam and the fact that we cook food at temperatures of about 120°С means that we can cook our food faster and use less energy to achieve the same result as we would with boiling or cooking in the oven.
We keep the mass and the volume of the contents of the pressure cooker more or less constant by preventing most of the steam from escaping. This ensures that the specific volume also stays somewhat constant. As we discussed earlier, if one among either the pressure, the temperature, or the specific volume is kept constant, then the magnitude of the other two variables depends on each other. Thus, if we increase temperature, as we do during pressure cooking, then the pressure inside increases also. Eventually we would reach the temperature-pressure balance, such that further increase in external temperature would result in evaporation. This is the maximum temperature that can be reached for the given specific volume and pressure. This is when we lower the heat to keep the temperature and the pressure constant for the remaining cooking time.
Using a pressure cooker is not only energy-efficient. It also reduces cooking times, as we mentioned before, and thus heats the kitchen a lot less, which is useful during the warm seasons. It can also be argued that pressure cooking is a healthy option because it does not require additional oil as does frying or stir-frying.
Artikel ini ditulis oleh Kateryna Yuri.
Anda kesulitan menerjemahkan satuan pengukuran ke bahasa lainnya? Bantuan tersedia! Posting pertanyaan Anda di TCTerms dan Anda akan mendapatkan jawaban dari penerjemah teknis berpengalaman dalam hitungan menit.
Page 12
1 liter/kilogram [L/kg] = 0,001 meter³/kilogram [m³/kg]
The working fluid (steam) rotates the turbine of this gas-powered power station as it expands through it.
Heating, Ventilating, and Air Conditioning Technology
Pressure, Temperature, and Specific Volume
Specific Volume in Pressure Cookers
Overview
Refrigeration cycle. Step 1. The hot refrigerant, which was compressed by the compressor is cooled by outside air flow and condensed in this heat exchanger of a window air conditioner
Specific volume indicates volume per unit of mass — a property of substances and materials that is often used in thermodynamics. It is the inverse of density. To find specific volume we divide volume by mass. For gases it can also be found by using pressure, temperature, and the molecular mass. While volume per unit of mass is more common, sometimes specific volume may refer to volume per molecular weight. We can usually tell from the context which specific volume is used. The units for the specific volume by mass and specific volume by moles are different, so it is also possible to tell which specific volume is meant by looking at the units. Specific volume per mass is measured in m³/kg, L/kg, or ft³/lb and units derived from those, while molar volume is measured in m³/mol, and units derived from it. In some cases the latter is called molar volume or molar specific volume.
Applications
When we compare solids, liquids, and gases we will note that it is much easier to change density and specific volume for gases. Incidentally, while density is more commonly used when talking about liquids and solids, specific volume is more often used when working with gases. If we talk about systems that have both gas and a liquid within them, it is common to use specific volume when talking about both states of a substance.
Refrigeration cycle. Step 2. The cooled refrigerant in the liquid form is routed through the expansion valve and enters the evaporator heat exchanger shown in this picture. The warm room air is blown across the cold evaporator and is cooled there
Two-Phase Systems
Systems that have within them a substance in two different states of matter (liquid and gas, or liquid and solid) are known as two-phase systems. A mix of ice and water in a cup is one example. A system that has both liquid and vapor of this liquid in it, for example in a boiler of a gas-powered power station, or in a nuclear reactor, or in an air conditioner is another. We may want to observe how such a system behaves when temperature or pressure changes, in particular — whether the amount of substance in one phase increases or decreases as these parameters change. This is where we would use specific volume. To quantify properties of a two-phase system in general it is also convenient to use specific volume.
First let us consider some examples of two-phase systems and how they are used in our daily lives and in technology. After that we will discuss the relevance of specific volume.
Heating, Ventilating, and Air Conditioning Technology
Refrigeration cycle. Step 3. The gaseous refrigerant from the evaporator enters the compressor, is compressed there to a higher pressure and then enters the condenser heat exchanger and the refrigeration cycle is repeated
In many cases heating, ventilating, and air conditioning (HVAC) installations consist of a two-phase system. When the heating is on, it is sometimes the case that water is heated to produce steam, and the hot steam is then circulated through the pipes of the system to heat the room. Hot water is often used in many systems to carry the heat along the pipes. Some of these HVAC systems use boilers, which are heating devices. The water inside them is heated by burning some fuel, often the fossil fuel. Coal is commonly used, and so is natural gas.
When the air conditioning is in operation the air in the room is cooled by a substance called refrigerant that alternates between a gas and a liquid state. Initially this substance is cooled as a gas in the heat exchanger called condenser. When it cools, it condenses, meaning that it becomes a liquid. It is then sent by a compressor through the system to cool the room, and in this process it evaporates in another heat exchanger called evaporator and becomes a gas again, as it warms up. It is then returned to the first heat exchanger (condenser) to repeat the process.
Outside unit of a mini-split air conditioner system
Changing from liquid to gas requires much energy. During the cooling process the system absorbs energy from the room to heat the refrigerant, and in the process cools the room. The condenser in the air conditioner cools the gas by releasing the energy outside.
Kitchen and industrial refrigerators work using the same principle. Some of the HVAC systems work as a single unit, and some are a combination of a separate heater and an air conditioner.
Solar thermal collectors are used for heating
Solar Thermal Collectors
Thermal collectors within solar panels, in particular the flat plate collectors, work in a somewhat similar fashion as well. Solar energy enters the system through the front panel and heats air or liquid such as water or antifreeze. This thermal heat is used for heating or to provide hot water.
Heat pipes are highly efficient thermal conductors due to very high heat transfer during boiling and condensation of a liquid inside a heat pipe
Heat Pipe Systems
In a heat pipe system the process is similar to the air conditioner, except that it is not air that is cooled but surfaces made from various solid materials such as metals. The heat of these surfaces vaporizes the liquid in the heat pipe. The rest of the process is similar, with the gas condensing when cooled, and then returning back into the system. Some examples of cooling agents are helium, alcohol, and mercury. These systems are often used in electronic components especially in computers, to cool parts that are subject to rapid or extreme heating. They are also used in space in very extreme conditions.
How Two-Phase Systems Work
In a two-phase system there is usually a set of conditions in which both states can coexist within the system. If the properties of a substance is outside of the range of these conditions, then the substance can only be in one state, as we discuss in more detail below.
When we work with a two-phase system, it is the change in pressure, not in specific volume, that causes the temperature changes. However, this pressure change also changes the specific volume. In some situations we have a special case where the pressure and the temperature are constant, but the specific volume is changing. This happens if we have a system with constant pressure, at a temperature that allows two phases of the same substance to co-exist. In this case, once the system reaches this temperature, if it stays constant, the liquid within the system starts turning into gas and the specific volume increases as a result. Of course in this case the total volume of the system changes as well, so this is only possible if the system is flexible enough to accommodate that. In systems that have a set volume and mass and do not allow the specific volume to change, as we see in the example of a pressure cooker, the process is different. In our system the increase in specific volume will continue until the liquid evaporates and the system reaches equilibrium again.
The design of boilers and steam turbines used in power plants like the gas-powered plant shown in this picture requires a detailed understanding of two-phase flow heat transfer and pressure drop behaviour.
We just looked at what happens if we keep the pressure constant. Now let us consider the case when the temperature is constant and we increase the pressure. For each substance there is a specific range of pressure for which the substance can only exist in gas state. There is also a range of pressure for which the gas and the liquid state of the substance can exist together in the same system. As we change pressure, specific volume of the substance also changes.
Temperature also has a specific value above which the substance cannot coexist as a liquid and a gas in one system. This value for temperature is called critical temperature, and the value for pressure is called critical pressure. In thermodynamics, the critical temperature and the critical pressure combined are called a critical point.
In thermodynamics pressure, temperature, and specific volume are three values that form a special relationship. It is convenient to use them for describing a thermodynamic system because they are easy to measure. As we have discussed earlier, as long as we work with a single phase substance, a change in pressure or a change in temperature causes a decrease or an increase in specific volume. It depends on the substance, but for most gases increase in pressure means a decrease in specific volume, as long as we keep the temperature constant. On the other hand, increase in temperature results in increase in specific volume if the pressure is constant. This relationship means that if we control the specific volume, we can also control either pressure or temperature. This is the principle used in the pressure cooker.
In a pressure cooker, the boiling point of water increases up to 121 °C (250 °F) at the sea level at a pressure of 1 bar or ~15 psi above the existing atmospheric pressure
Specific Volume in Pressure Cookers
A pressure cooker is generally filled with food which is in liquid state. Often there is also some food in a solid state, but liquid is necessary for this type of cooking to work. When the lid is closed, the vapor cannot escape from the pressure cooker chamber except through the steam vent with the pressure regulator on it. Therefore during the cooking process the total volume is the same. The goal for pressure cooking is to cook the food inside at a higher temperature without allowing too much liquid to evaporate. This shortens the cooking time. We do need to generate some steam, however, because the additional goal that we try to achieve with a pressure cooker is to use hot steam for cooking. Steam has a much higher heat capacity than air, meaning that it is more efficient at storing energy. This is why it is used in pressure cooking. The energy efficiency of steam and the fact that we cook food at temperatures of about 120°С means that we can cook our food faster and use less energy to achieve the same result as we would with boiling or cooking in the oven.
We keep the mass and the volume of the contents of the pressure cooker more or less constant by preventing most of the steam from escaping. This ensures that the specific volume also stays somewhat constant. As we discussed earlier, if one among either the pressure, the temperature, or the specific volume is kept constant, then the magnitude of the other two variables depends on each other. Thus, if we increase temperature, as we do during pressure cooking, then the pressure inside increases also. Eventually we would reach the temperature-pressure balance, such that further increase in external temperature would result in evaporation. This is the maximum temperature that can be reached for the given specific volume and pressure. This is when we lower the heat to keep the temperature and the pressure constant for the remaining cooking time.
Using a pressure cooker is not only energy-efficient. It also reduces cooking times, as we mentioned before, and thus heats the kitchen a lot less, which is useful during the warm seasons. It can also be argued that pressure cooking is a healthy option because it does not require additional oil as does frying or stir-frying.
Questo articolo è stato scritto da Kateryna Yuri.
Hai trovato delle difficoltà nel tradurre un’unità di misura in un’altra lingua? Ti possiamo aiutare! Posta la tua questione nei TCTerms e riceverai le risposte di esperti traduttori tecnici in pochi minuti.
Gallery Konversi Kg Ke M3
 How To Convert Kn M3 To Gm Cm3
How To Convert Kn M3 To Gm Cm3
 How To Convert Kg Cm2 To Kg M2 Pressure Converter
How To Convert Kg Cm2 To Kg M2 Pressure Converter
Lpg Gas Unit Conversion Values Kg Litres Mj Kwh M
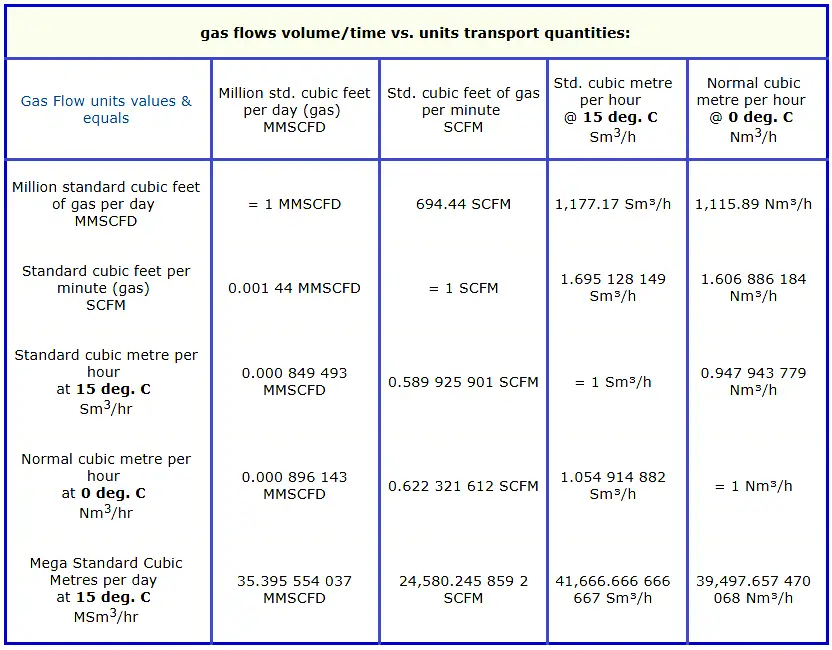 Gas Flow Formulas Mmscfd Conversions Instrumentationtools
Gas Flow Formulas Mmscfd Conversions Instrumentationtools
 1 Liter Berapa Kg Berikut Jawaban Dan Penjelasannya
1 Liter Berapa Kg Berikut Jawaban Dan Penjelasannya
 Latihan Soal Energi Kinetik Gas
Latihan Soal Energi Kinetik Gas
How To Convert A Ppm To Ml Quora
1 Kg Berapa Liter Ukuran Dan Satuan
 Konversi Satuan Internasional Si Kunci Jawaban Kurikulum
Konversi Satuan Internasional Si Kunci Jawaban Kurikulum
 How To Convert M3 To Kg For Lpg
How To Convert M3 To Kg For Lpg
 How To Quickly Convert Pounds To Ounces Grams Kg In Excel
How To Quickly Convert Pounds To Ounces Grams Kg In Excel
 1 Liter In M3 Litre 2019 10 05
1 Liter In M3 Litre 2019 10 05
 English Ari Armaturen Gmbh Co Kg
English Ari Armaturen Gmbh Co Kg
 Konversi Satuan Pengertian Panjang Massa Waktu Luas Dan
Konversi Satuan Pengertian Panjang Massa Waktu Luas Dan

 How To Quickly Convert Pounds To Ounces Grams Kg In Excel
How To Quickly Convert Pounds To Ounces Grams Kg In Excel
 Membuat Sendiri Aplikasi Konversi Liter Ke Kg Kilogram
Membuat Sendiri Aplikasi Konversi Liter Ke Kg Kilogram
 Converting Occupational Exposure Limits From Mg M3 To Ppm
Converting Occupational Exposure Limits From Mg M3 To Ppm








0 Response to "Konversi Kg Ke M3"
Post a Comment